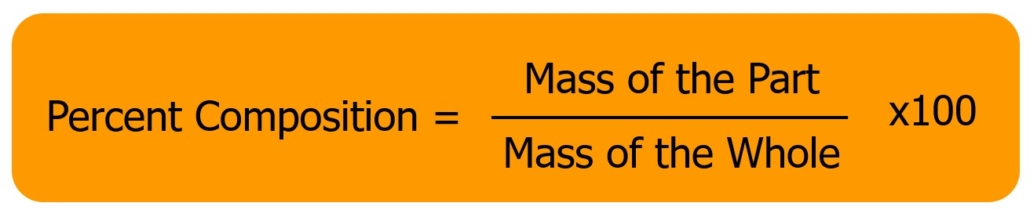Determination of the Percent Mass of Carbon Dioxide in Pop Rocks®
Objectives
- Apply knowledge of scientific theories to problem-solving applications.
- Draw conclusions based on experimental data.
- Identify sources of error and explain their impact on experimental data.
- Calculate percent composition.
- Determination of the Percent Mass of Carbon Dioxide in Pop Rocks®
Related Textbook
Please read sections 6.5 and 6.6 of your textbook before beginning this activity. The textbook provides terms, concepts, and other important background information to help you complete this assignment.
Introduction
Pop Rocks® was created by General Mills in 1956 but wasn’t released to the public until 1975. Like most hard candies, this candy is made from sugar, corn syrup, water, and flavoring. Unlike other hard candies that melt in your mouth as you eat them, Pop Rocks® releases carbon dioxide as it dissolves in the mouth. This occurs because, during production, the sugar mixture is allowed to cool in the presence of carbon dioxide gas at a pressure of about 600 psi. This causes carbon dioxide bubbles to form in the candy as it cools. The carbon dioxide is then released as the candy dissolved in the mouth, causing the popping that occurs when Pop Rocks® are consumed.
In the late 1970s and early 1980s, Pop Rocks® came under investigation out of concern that consuming them in combination with soda would cause the person’s stomach or head to explode. An FDA hotline was created so concerned parents could call in with questions. In reality, consuming Pop Rocks® and soda causes no harm. General Mills started a campaign to dispel these rumors, including taking out ads in 45 major publications and writing 50,000 letters to principals across the US. (FAQ) Despite all of these efforts, this urban legend of Pop Rocks® is still tested by kids each year.
In this experiment, we will calculate the percent composition of carbon dioxide in Pop Rocks®. The amount of carbon dioxide in Pop Rocks® will be experimentally determined by dissolving a known amount of Pop Rocks® in water, releasing the carbon dioxide. You will measure the change in mass to determine the amount of carbon dioxide in the Pop Rocks®, then calculate the percent mass of carbon dioxide in Pop Rocks® using the formula below:
Figure 1.0 – Percent Composition Formula
In this experiment, the mass of the part is the carbon dioxide, since that is the part we are experimentally determining. The mass of the whole is the sample of Pop Rocks® used for each trial of this experiment. In lecture, we have been calculating the percent composition for elements in a compound. This is the same concept we are using here. In lecture, the part is the atomic mass of the single element we are considering. The mass of the whole is the atomic mass of the entire compound the element is a part of.
You will organize your data for this lab into a data table. Click below for a copy of the data table you will need for this lab. It will download to your computer. If you have trouble downloading it, check to ensure blockers are disabled.
Safety Concerns
There are no safety hazards in this experiment.
Do not eat the Pop Rocks®. This candy is considered a laboratory chemical and should not be consumed as a part of this lab.
Experimental Procedure
Chemicals and Supplies
4 packages of Pop Rocks® candy of the same flavor
250-mL beaker
100 Graduated Cylinder
Glass Stir Rod
Digital Balance
Weight boats (cupcake liners)
DI Water
1. Obtain the mass of two packages of Pop Rocks®. Record this information on the data table under Trial 1.
NOTE: To properly measure the mass of the Pop Rocks®, place a weigh boat on the digital balance and “TARE” the balance according to the directions provided with your balance. This tells the balance to ignore the mass of the weigh boat and only measure the mass of the chemical. Next, add the Pop Rocks® to the weigh boat and record the mass on the data table.
2. Measure 100mL of DI water using the graduated cylinder. Place the DI water in a 250mL beaker. Obtain the mass of the beaker and water. Record this information on the data table under Trial 1.
3. Place the Pop Rocks® in a 250mL beaker. Stir the beaker with the glass-stirring rod until the candy is fully dissolved.
4. Obtain the final mass of the beaker, DI water, and dissolved Pop Rocks®. Record this information on the data table under Trial 1.
Take a photo of the beaker on the balance with the beaker on it from step 4. A piece of paper that includes the trial number, your name, CHEM 1000, and the semester you are taking this class (for example, if you are taking this during the fall semester in 2023, write “Fall 2023” on the paper) should also appear in this photo. This information must be handwritten, legible, and large enough to be clearly read on the piece of paper. The assignment tied to this laboratory will not be graded unless this photo has been submitted to the assignment folder on Brightspace along with the rest of the assignment for this experiment.
5. Repeat steps 1 through 4 of this experiment, recording the information on the Data Table under Trial 2. Be sure also to take a photo for this trial, as outlined in the box at the end of step 4. (4)
Waste Disposal
- Pour all chemicals down the drain, flushing the sink thoroughly with water.
- Wash any other glassware used with soap and water once the experiment is complete.
Assignment
Now that you have completed the experiment and recorded your data in the data table:
- Use the data from your experiment to complete the calculations included at the bottom of the data table.
- Answer the post-lab questions included below. All calculations and questions MUST be handwritten. Typed submissions for this assignment will not be accepted. Please be sure to number your answers so they are easy to follow.
- Submit the data table and the post-lab questions to the assignment folder on Brightspace along with your photo from this experiment. You will receive an automatic zero for this assignment if the experimental photo or data are missing from your submission.
- This assignment is worth 10 points.
Post-Lab Questions
- Compare the two trials of this experiment. Is the percent of carbon dioxide in each sample similar? Why or why not? What does this tell you about the amount of carbon dioxide in each packet of Pop Rocks®? Is the manufacturer consistent in their production? Support your conclusion with your experimental data. (3 points)
- You accidentally knocked the side of the beaker in Trial 1 and some of the solution splashes out onto the tabletop, causing the final mass of the beaker and its contents to be lower than it should be. Specifically explain how this would impact your percent composition of carbon dioxide in your Pop Rocks® Would the amount of carbon dioxide you calculated be higher or lower than it should be? Explain your reasoning. (Hint: It may help you to do a sample calculation simulating this error to determine the impact.) (2 points)
- A 2.67g sample of potassium nitrate is experimentally determined to contain 1.03g of potassium and 1.27g of oxygen. What is the percent composition of each of the elements in this sample of potassium nitrate. You must show ALL of your work to receive credit for this problem. (3 points)
References
(1) “Are Pop Rocks Dangerous?” YouTube, Brain Stuff – How Stuff Works, 3 Mar. 2014, www.youtube.com/watch?time_continue=58&v=q_htMUHHz1s.
(2) “FAQ.” Pop Rocks, 25 Oct. 2018, www.pop-rocks.com/f-a-q/.
(3) “How Does Pop Rocks Candy Work?” How Stuff Works, 8 Mar. 2018, https://science.howstuffworks.com/innovation/science-questions/question114.htm.
(4) Katz, David. “Determination of the Volume of CO2 in Pop Rocks.” Chymist.com, 2009, www.chymist.com/CO2%20in%20Pop%20Rocks.pdf.
This page was created on July 11, 2023, and was last updated on August 3, 2023.
©2023 Catherine Haslag. All Rights Reserved.