Chemical Properties of Matter
Objectives
- Distinguish between a physical and a chemical change.
- Determine if a process is exothermic or endothermic.
- Draw conclusions based on experimental data.
Related Textbook
Please read sections 3.5 and 3.6 of your textbook before beginning this activity. The textbook provides terms, concepts, and other important background information that will help you succeed on this assignment.
Chemical Properties and Changes of Matter
During a chemical reaction, the reactants undergo a chemical change and become new substances. If a chemical change has occurred, one or more of the following will be observed (click the link provided to observe an example of each type of reaction:
- Formation of a gas – You will see this as the formation of bubbles. (illustrated in Figure 1.0)
- Temperature Change – This example also shows gas being produced in the first video of Figure 1.0. Thermometers are used to measure a temperature change.
- Formation of a precipitate – precipitate is the chemistry term used when a solid forms during a chemical reaction. This solid can only disappear by way of a chemical process. This is not a phase change, which is a physical process. Be sure to indicate a precipitate formed if this occurs in a chemical reaction. This is the proper term for this type of chemical change. (illustrated in Figure 1.0)
- Permanent Color Change (illustrated in Figure 1.0)
- pH Change – pH changes (changes in how basic or acid something is) can’t be seen without using a chemical or other piece of laboratory equipment. You must use an indicator to determine these changes. Indicators include litmus paper, pH paper, a pH meter, or a chemical indicator such as a universal indicator or phenolphthalein. To learn more about what pH is, the pH scale, and how we measure pH in the lab, watch the videos in Figures 2.0 and 3.0. This video refers to bases by calling them alkaline. The two terms are interchangeable (base=alkaline).
- Light is produced. An example is when a candle burns or fireworks produce their brilliant colors.
- Odor Change – Sorry, I can’t show you an example of this via video, but think about any time you bake. The house smells like what you are baking. THAT is an example of the chemical reaction occurring in the item you are baking. Cooking and baking are examples of chemical reactions. When checking for an odor change, DO NOT stick your nose in the container and inhale deeply. This can cause you to inhale something nasty. You want to waft towards your nose to check for an odor change. Figure 4.0 demonstrates how to waft.
If you did not observe at least one of the above occur when two chemicals are mixed together, then a new substance was not formed, and chemical reaction didn’t occur. It is possible to mix chemicals together and not have a chemical reaction occur.
It helps to record our observations during a chemical reaction and specific information regarding the change to determine if a chemical reaction occurred. This includes but is not limited to:
- The color of the solution or if a precipitate forms.precipitate (if the solution is colorless, be sure to note it)
- The type of odor detected (ammonia, fruity, acrid, etc.)
CAUTION! Don’t forget to waft when checking for an odor change.
- The amount of temperature change observed
- If the temperature of a reaction decreases, the reaction took in heat from its surroundings and is called an endothermic reaction. The reaction vessel will feel colder to the touch.
- If the temperature of a reaction increases, heat was produced during the reaction and is called an exothermic reaction. The reaction vessel will feel warmer to the touch.
- The starting and ending pH – this is measured using the pH paper you purchased.
- The type of gas produced (additional testing may be required and will not be conducted in this experiment).
Figure 1.0 (above) is a playlist containing four videos on chemical changes that involve the evolution of gas, a color change, and the formation of a precipitate.
Figure 2.0 (above) explains pH and how chemical indicators are used to visually determine when a change in pH has occurred. (1)
Figure 3.0 (below) is an example of the use of phenolphthalein to determine the change in pH. Phenolphthalein and other chemical indicators contain a class of chemicals called a chromatophore which changes color with pH.
Figure 4.0 (below) demonstrates how to waft. (2)
The information observed during a chemical change can help determine what products were actually formed during the reaction. More than one of these changes can occur during a chemical reaction. It is important to note ALL of the evidence observed during a chemical reaction on the datasheet provided for this experiment.
Is it possible to have a physical and a chemical change occur at the same time?
It is possible to have a physical change and a chemical change happen at the same time; however, it will ONLY be a chemical change if one of the seven changes outlined above (formation of a precipitate or gas; change in pH, odor, temperature, or color; or production of light) are observed.
What is the difference between a phase change and a precipitate?
- A phase change can go back and forth. For instance, water can freeze and become ice or melt and become water. This phase change can go back and forth indefinitely simply by changing the temperature of the soluion. This is evidence of a physical change because ice and water both have the same chemical formula,
- A precipitate is a solid that won’t turn to another phase by physical means. For instance, when cooking an egg (see Figure 5.0), heat is applied to the egg, which causes a reaction. The egg changes color (the yellow yolk becomes a different shade of yellow and the clear albumin turns white), but a precipitate also forms because the egg becomes a solid. This is NOT a phase change because the egg can’t return to being a liquid with a temperature change. If you cool the egg down (no matter how cold the egg gets), the egg will still be solid.
Figure 5.0 – Frying an egg.
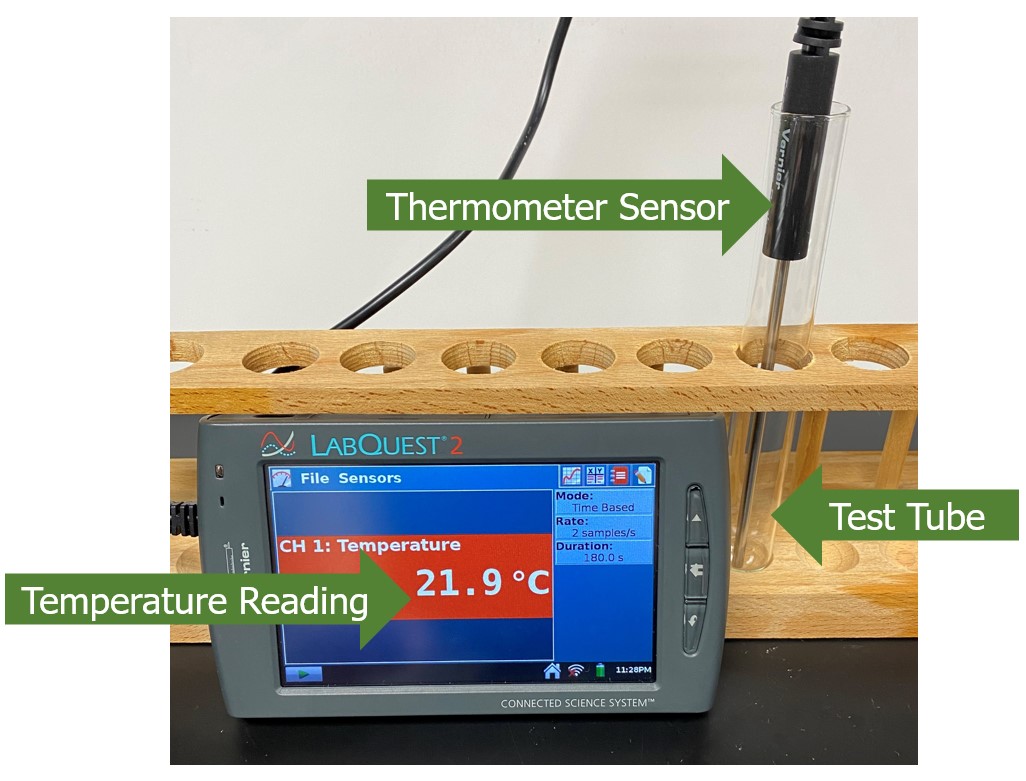
Figure 6.0 – Arrangement of equipment in the videos for this experiment.
NOTE: You will not actually be performing parts A to G of this experiment. Instead, you are viewing the reactions via video links provided by your instructor. These videos are provided in the body of the experimental procedure.
Be sure to note the reactant’s appearance before in the test tube before the reactants are combined. I recommend pausing the video and writing down a brief description of the contents of the test tube before the second reactant is added. The change in what you see before and after will help you determine if a chemical reaction has occurred.
When the reactants are combined, a temperature probe will be placed in the test tube. The screen that will be visible on the video’s left side indicates the reaction’s temperature (see Figure 6.0). Be sure to record the beginning and final temperature of each reaction. If a temperature change of more than 1°C is observed, assume there was a temperature change for the reaction.
You will be performing reactions H and I at home in your laboratory. A copy of the experimental procedure for this lab is provided below. Please read through it to understand how the reactions are being conducted.
Record your observations on the data sheet provided. Based on your observations, determine if a chemical reaction occurred and record your conclusion on the data sheet provided. Record ALL evidence of a chemical reaction. Some reactions may have more than one, and this is fine. Report them all. Please be advised some of the chemicals may not result in a reaction. In this case, write “No reaction occurred.”
I selected reactions that would be easy to see on video. It will be clear if a reaction occurred or not. You should be easily able to observe all evidence of a chemical reaction on each video provided for Reactions A to G.
Since reactions A through G of this experiment are being conducted using a video, you cannot observe any odor changes for the reactions. Just record the observations you can make from the video.
You will organize your data for this lab into a data table. Click below for a copy of the data table you will need for this lab. It will download to your computer. If you have trouble downloading it, check to ensure blockers are disabled.
Chemical Properties of Matter Data Table
Experimental Procedure
I listed the steps for Reactions A to G below. I took These general steps to complete each reaction in the corresponding videos. These videos are compiled in a playlist as shown in Figure 7.0.
A. Reaction of Magnesium and Acid (Video)
- Place approximately 2mL of HCl in a test tube.
- Add a piece of magnesium to the acid in the test tube.
- Record your observations on the data sheet.
B. Reaction of Sodium Chloride and Calcium Nitrate (Video)
- Place approximately 1mL of each reactant in the same test tube.
- Record your observations on the data sheet.
C. Reaction of Calcium Chloride and Sodium Carbonate (Video)
- Place approximately 1mL of each reactant in the same test tube.
- Record your observations on the data sheet.
D. Reaction of Sodium Hydroxide and Hydrochloric Acid (Video)
- Place approximately 1mL of sodium hydroxide in the test tube.
- Add a few drops of phenolphthalein. Record what happens.
- Add approximately 1mL of hydrochloric acid.
- Record your observations on the data sheet.
E. Reaction of Silver (I) Nitrate and Sodium Chloride (Video)
- Place approximately 1mL of each reactant in the same test tube.
- Record your observations on the data sheet.
F. Reaction of Lead (II) Nitrate and Potassium Iodide (Video)
- Place approximately 1mL of each reactant in the same test tube.
- Record your observations on the data sheet.
G. Reaction of Potassium Iodide and Sodium Chloride (Video)
- Place approximately 1mL of each reactant in the same test tube.
- Record your observations on the data sheet.
Figure 7.0 – Videos for Reactions A to G.
PLEASE NOTE: The order of the videos may not be the order of the chemical reactions on the data table. Before recording any observations, verify the reactants stated in each video with the correct reaction on the data table.
Materials Used in Reactions H and I
Acetic Acid (Vinegar), HC2H3O2
Sodium Bicarbonate (Baking Soda), NaHCO3
Sucrose (Sugar), C12H22O11
50mL Beaker
100mL beaker
50mL Graduated Cylinder
Digital Thermometer
Digital Balance
Paper Cupcake Liners (Weigh boats)
Spatula
Glass Rod
pH paper
Paper towels
H. Reaction of Sodium Bicarbonate and Acetic Acid (Performing at Home)
- Obtain 1g of sodium bicarbonate and place it in a 50mL beaker.
NOTE: To properly measure this amount of sodium bicarbonate, place a weigh boat on the digital balance and “TARE” the balance according to the directions provided with your balance. This tells the balance to ignore the mass of the weigh boat and only measure the mass of the chemical. Next, use a spatula to add 1g of sodium bicarbonate to the weigh boat.
It is ok if you measure slightly more or less than 1g of the chemical. Just be sure to write down exactly how much of the chemical you use. Write down every number that appears on the balance, even if it’s a zero. This is true of all of the experiments we will conduct in this course.
- Using a 50mL graduated cylinder, measure 25mL of acetic acid. Be sure to measure to the bottom of the meniscus as described in the Physical Properties of Matter experiment.
- Determine the pH of the solution by following the directions provided in the video titled “How to Determine the pH of a solution” posted with this experiment on D2L. Record this information on the data table.
- Record the initial temperature of the acetic acid on the Data Sheet.
- Place the thermometer in the 50mL beaker.
- Pour the acetic acid into the beaker. Carefully swirl the beaker or stir the solution with the glass rod. Record the temperature of the solution and your observations on the Data Sheet.
- Determine the new pH of the solution. Record this information on the data table.
NOTE: The temperature will either go up or down. Record either the highest observed temperature (if it increases) or the lowest observed temperature (if it decreases).
I. Reaction of Sucrose and Acetic Acid (Performing at Home)
- Obtain 1g of sucrose (sugar) and place it in a 100mL beaker.
NOTE: To properly measure this amount of sucrose (sugar), place a weigh boat on the digital balance and “TARE” the balance according to the directions provided with your balance. This tells the balance to ignore the mass of the weigh boat and only measure the mass of the chemical. Next, use a spatula to add 1g of sodium bicarbonate to the weigh boat.
It is ok to measure slightly more or less than 1g of the chemical. Write down exactly how much of the chemical you use. Write down every number that appears on the balance, even if it’s a zero. This is true of all of the experiments we will conduct in this course.
- Using a 50mL graduated cylinder, measure 25mL of acetic acid. Be sure to measure to the bottom of the meniscus as described in the Physical Properties of Matter experiment.
- Determine the pH of the solution by following the directions provided in the video titled “How to Determine the pH of a solution” posted with this experiment on Brightspace. Record this information on the data table.
- Record the initial temperature of the acetic acid on the Data Sheet.
- Place the thermometer in the 50mL beaker.
- Pour the acetic acid into the beaker. Carefully swirl the beaker or stir the solution with the glass rod. Record the temperature of the solution and your observations on the Data Sheet.
- Determine the new pH of the solution. Record this information on the data table.
NOTE: The temperature will either go up or down. Record either the highest observed temperature (if it increases) or the lowest observed temperature (if it decreases).
Waste Disposal
- All solutions from this lab can be disposed of down the sink. Flush the sink with warm water after disposing of the chemicals.
- Wash any other glassware used with soap and water once the experiment is complete.
- The used pH paper can be disposed of in the trash.
- Since most of this experiment is complete via video, there is not experimental photo to upload for this lab.
Assignment
Now that you have completed this experiment and recorded your data:
- Upload a copy of your data sheet to the assignment folder. Your data table can be neatly handwritten or typed using a program such as MSWord or Google Docs. If using a word processing program, please be sure to save your file as a .docx or .pdf file prior to submitting it to Brightspace. The associated electronic activity will open once you have uploaded your data sheet to the assignment folder.
- Complete the associated electronic activity provided on Brightspace. You will use the data and conclusions you drew in this experiment to complete the electronic activity.
References
(1) Fuse School. Indicators [Video]. YouTube. August 10, 2014. https://youtu.be/6ojbQakWI8A (Accessed August 10, 2023).
(2) Jason Ribblett. How to Waft Vapors. [Video]. YouTube. August 30, 2023. https://youtu.be/MXBtmKrVAL8 (Accessed August 10, 2023).
This page was created on August 3, 2023, and was last updated on August 15, 2023.
©2023 Catherine Haslag. All Rights Reserved.