The following is a list of references and resources for students and teachers regarding OA. I will update this list as I find new materials I use in my class or think would be useful to others.
Please note that many of the resources for educators can also be shared with students depending on how the teacher structures the discussion of OA in their course.
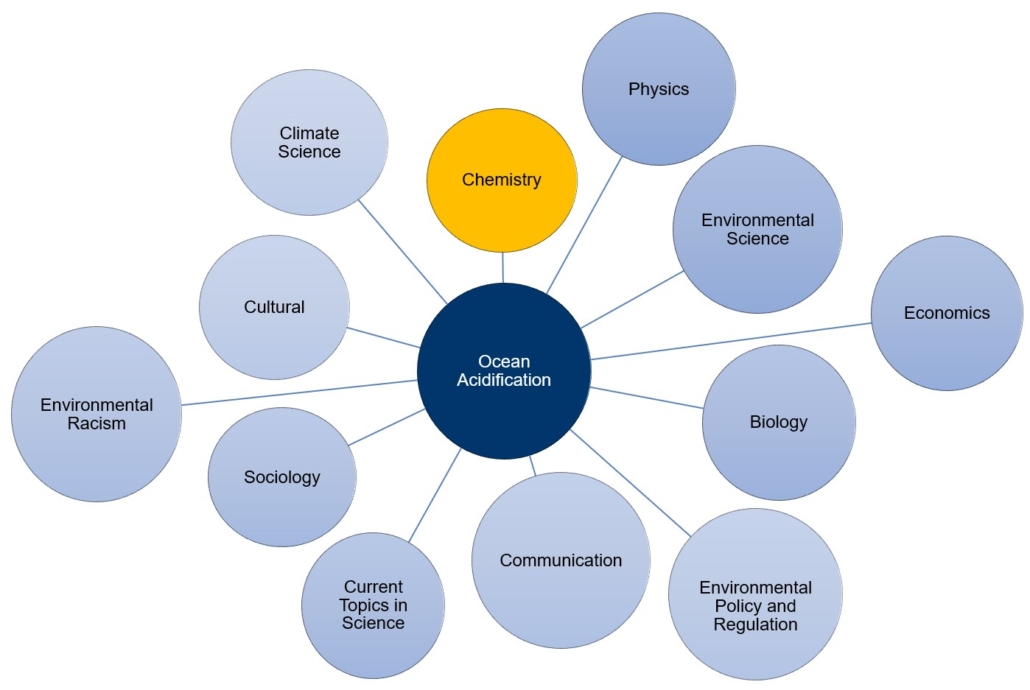
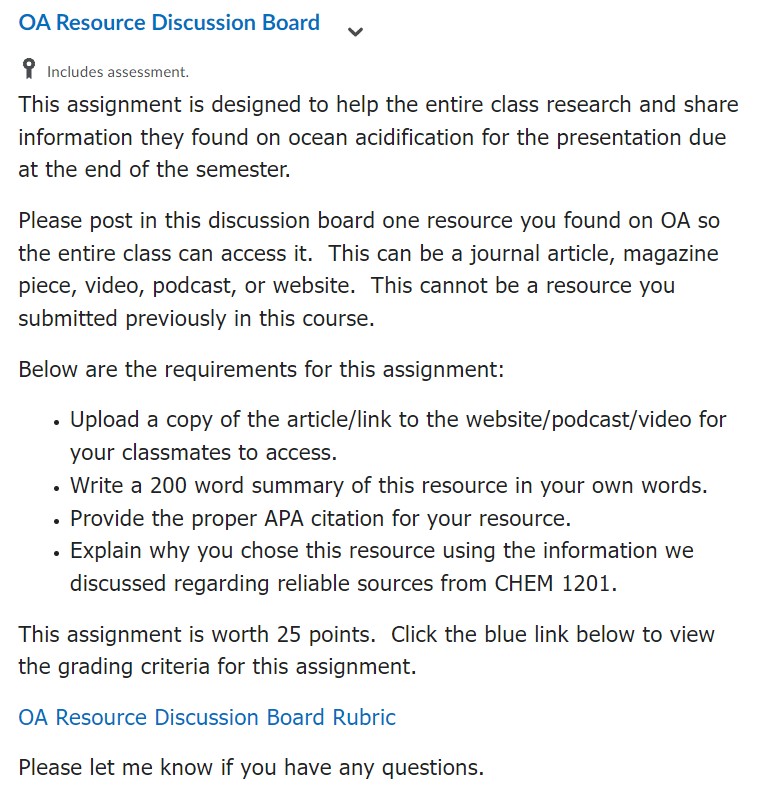
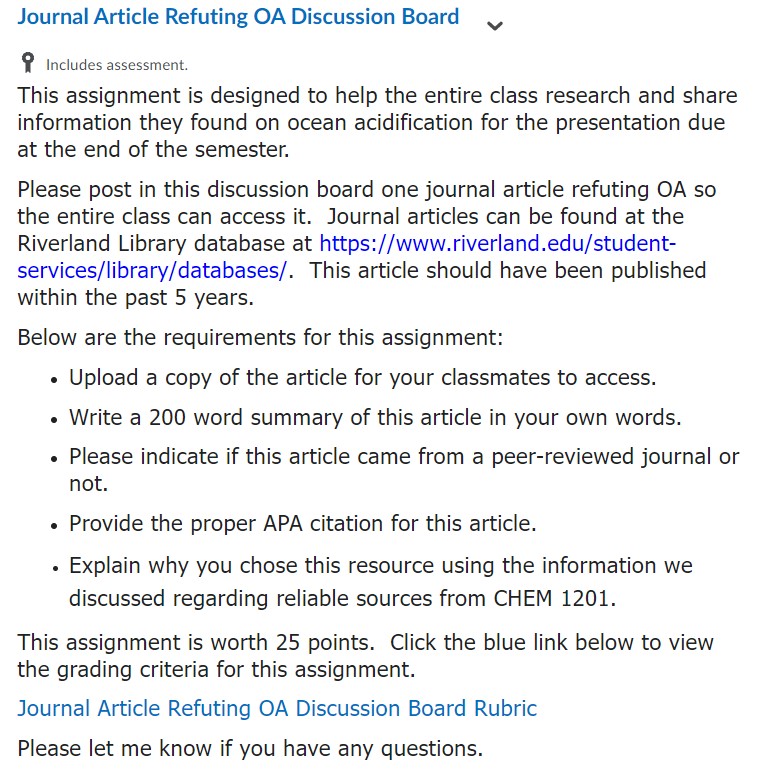
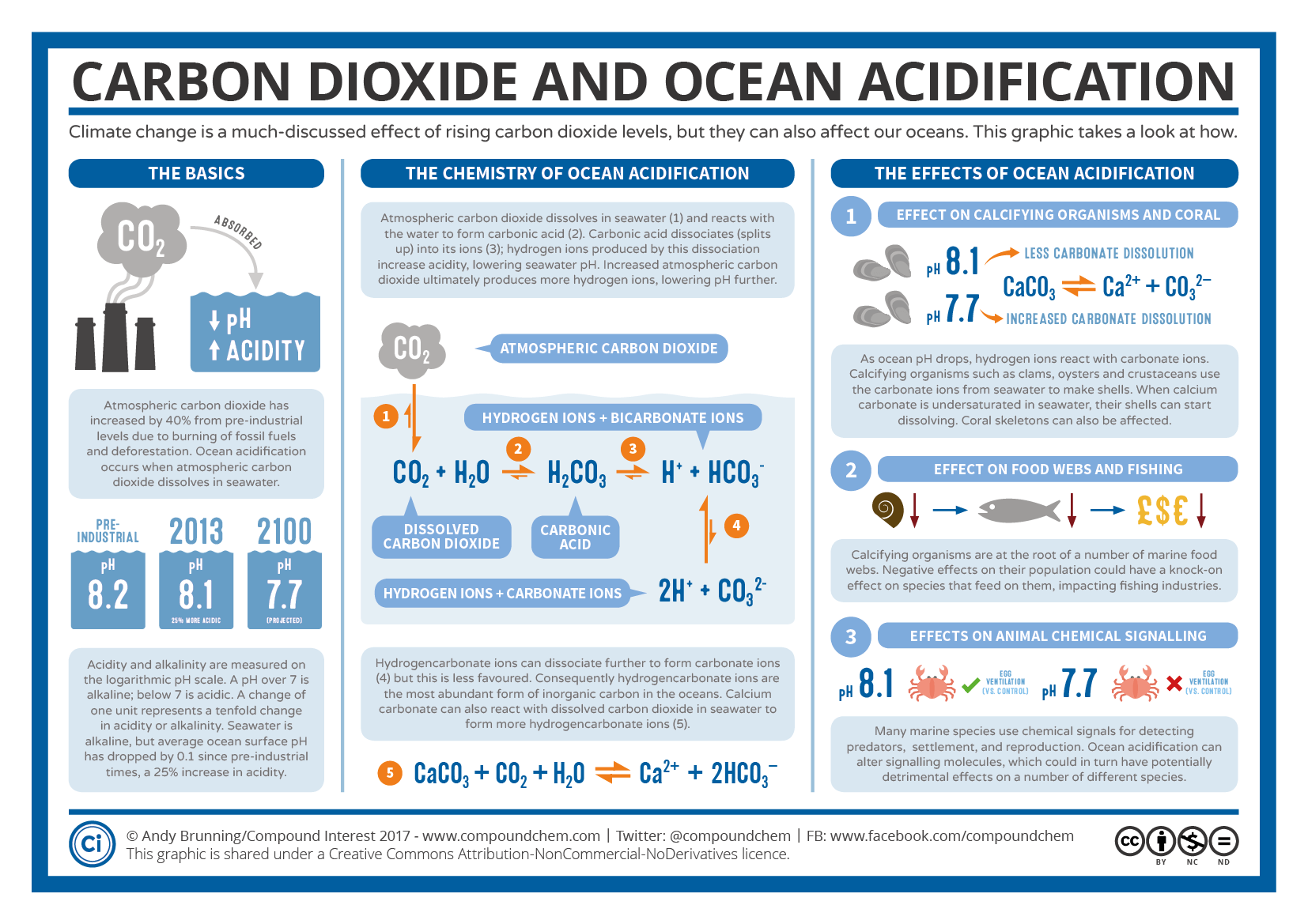
Resources for Educators
Action for Climate Emergency, (2016, April 7). What is Ocean Acidification? [Video]. YouTube. https://youtu.be/6SMWGV-DBnk
American Chemical Society. (2022). Ocean Chemistry. American Chemical Society Retrieved June 30, 2022. https://www.acs.org/content/acs/en/climatescience/oceansicerocks/oceanchemistry.html
Aubrecht, K. B. (2018). Teaching relevant climate change topics in undergraduate chemistry courses: Motivations, student misconceptions, and resources. Current Opinion in Green and Sustainable Chemistry, 13, 44–49. https://doi.org/10.1016/j.cogsc.2018.03.008
Bozeman Science, (2016, Dec 12). Ocean Acidification [Video]. YouTube. https://youtu.be/fgBozLCGUHY
Cooper, M. (2010). The Case for Reform of the Undergraduate General Chemistry Curriculum. Journal of Chemical Education, 87(3), 231–232. https://doi.org/10.1021/ed800096m
Cooper, M., & Klymkowsky, M. (2013). Chemistry, Life, the Universe, and Everything: A New Approach to General Chemistry, and a Model for Curriculum Reform. Journal of Chemical Education, 90(9), 1116–1122. https://doi.org/10.1021/ed300456y
Dickson, A.G. (2010). Seawater Carbonate Chemistry. U. Riebesell, V.J. Fabry, L. Hansson, & P. Gattuso (Eds.), Guide to Best Practices for Ocean Acidification Research and Data Reporting. (pp. 17-52). https://www.pmel.noaa.gov/co2/files/dickson_thecarbondioxidesysteminseawater_equilibriumchemistryandmeasurementspp17-40.pdf
Dunbar, R. (2010. Sept 13). Discovering Ancient Climates in Oceans and Ice [Video]. TED. https://www.ted.com/talks/rob_dunbar_discovering_ancient_climates_in_oceans_and_ice
Erickson, G., Crews, T. (2019). From Dissolution to Solution: New approaches to teaching ocean acidification. The Science Teacher (National Science Teachers Association), 86(5), 56–65.
Gorospe, K. D., Fox, B. K., Haverkort-Yeh, R. D., Tamaru, C. S., & Rivera, M. A. J. (2013). Engaging Students in the Pacific and Beyond Using an Inquiry-Based Lesson in Ocean Acidification. Journal of Geoscience Education, 61(4), 396–404. https://doi.org/10.5408/12-390.1
Halliday, C., & Hatton, T. A. (2021). Sorbents for the Capture of CO2 and Other Acid Gases: A Review. Industrial & Engineering Chemistry Research, 60(26), 9313–9346. https://doi.org/10.1021/acs.iecr.1c00597
Hibbard, L. (2019). Case Studies for General Chemistry: Teaching with a Newsworthy Story. Journal of Chemical Education, 96(11), 2528–2531. https://doi.org/10.1021/acs.jchemed.9b00420
International Atomic Energy Agency. (n.d.). Chemistry of Carbonic Acid in Water. International Atomic Energy Agency. (Retrieved November 14, 2021). http://www-naweb.iaea.org/napc/ih/documents/global_cycle/vol%20I/cht_i_09.pdf
McGrath, T. (2016, Feb 26). How Pollution is Changing the Ocean Chemistry [Video]. Ted. https://www.ted.com/talks/triona_mcgrath_how_pollution_is_changing_the_ocean_s_chemistry
Mitchell, M. J., Jensen, O. E., Cliffe, K. A., & Maroto-Valer, M. M. (2010). A model of carbon dioxide dissolution and mineral carbonation kinetics. Proceedings of the Royal Society. Mathematical, Physical, and Engineering Sciences, 466(2117), 1265–1290. https://doi.org/10.1098/rspa.2009.0349
Morse, J.W., Mackenzie, F.T. (1990). The CO2-Carbonic Acid System and Solution Chemistry. Developments in Sedimentology. (pp. 1-38). Elsevier. https://doi.org/10.1016/S0070-4571(08)70330-3.
NGSS Lead States. (2013). Next Generation Science Standards: For States, By States. https://www.nextgenscience.org/
NOAA. (2022). NOAA Ocean Acidification Program. (Retrieved July 14, 2022). https://oceanacidification.noaa.gov/Home.aspx
Oregon State University, (2016, Jan 21). Ocean Acidification – Changing Waters on the Oregon Coast [Video]. YouTube. https://youtu.be/7h08ok3hFSs
Oregon State University, (2018, Sept 4). Ocean Acidifications – Part 2, Solutions [Video]. YouTube. https://youtu.be/2KLT9vFVOmc
Pence, & Losoff, B. (2011). Going beyond the textbook: The need to integrate open access primary literature into the Chemistry curriculum. Chemistry Central Journal, 5(1), 18–18. https://doi.org/10.1186/1752-153X-5-18
Sparks, D.L. (n.d.). Carbonate Equilibria taken from Environmental Soil Chemistry. University of California at Davis SSC 102 – Soil Chemistry. https://lawr.ucdavis.edu/classes/ssc102/Section5.pdf
Sumter T.F., Owens, P. M. (2011). An approach to teaching general chemistry II that highlights the interdisciplinary nature of science. Biochemistry and Molecular Biology Education, 39(2), 110–116. https://doi.org/10.1002/bmb.20465
Treptow. (2010). Carbon Footprint Calculations: An Application of Chemical Principles. Journal of Chemical Education, 87(2), 168–171. https://doi.org/10.1021/ed8000528
VC3Chem Team. (n.d.). Visualizing the Chemistry of Climate Change. Retrieved July 14, 2022. https://www.vc3chem.com/
Versprille, A. N., & Towns, M. H. (2015). General Chemistry Students’ Understanding of Climate Change and the Chemistry Related to Climate Change. Journal of Chemical Education, 92(4), 603–609. https://doi.org/10.1021/ed500589g
Case Studies
Spain, D.D., Mendoza, V.M. (2020). An Investigation into Ocean Acidification. National Science Teachers Association. https://www.nsta.org/ncss-case-study/investigation-ocean-acidification
Terry, T.J. (2020). Equilibria in the Environment. National Science Teachers Association. https://www.nsta.org/ncss-case-study/equilibria-environment
Resources for Students
Belli, B. (2012). This Is Your Ocean on Acid. E Magazine. (Norwalk, Conn.), 23(3), 22–31.
NBC News Learn, (2020, May 1). Ocean Acidification [Video]. YouTube. https://youtu.be/XajNg6ARogw
NRDCflix (2009, Sept 17). Acid Test: The Global Challenge of Ocean Acidification. [Video}. YouTube. https://youtu.be/5cqCvcX7buo
Morello, L. (2010, Feb 22). Oceans Turn More Acidic Than Last 800,000 Years. American Scientific. https://www.scientificamerican.com/article/acidic-oceans/
Ogden, L. E. (2013). Marine Life on Acid. Bioscience, 63(5), 322–328. https://doi.org/10.1525/bio.2013.63.5.3
Petkewich, R. (2009, Feb 23). Off-Balance Ocean. C&E News. Volume 87, issue 8,pp 56-68.
Strong, A. L., Kroeker, K. J., Teneva, L. T., Mease, L. A., & Kelly, R. P. (2014). Ocean Acidification 2.0: Managing our Changing Coastal Ocean Chemistry. Bioscience, 64(7), 581–592. https://doi.org/10.1093/biosci/biu072
Welch, C. (2014, April 30). Sea Change: Vital Part of Food Web Dissolving. The Seattle Times. http://apps.seattletimes.com/reports/sea-change/2014/apr/30/pteropod-shells-dissolving/