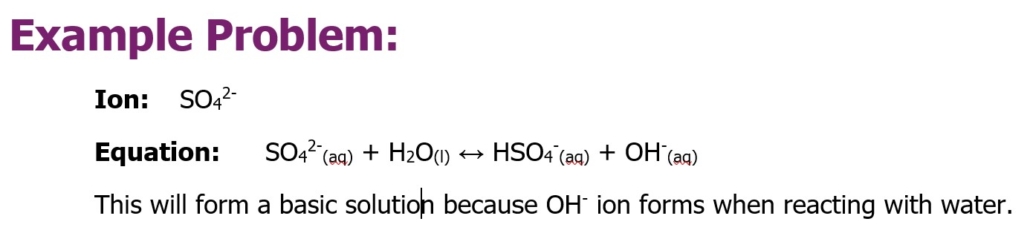Objectives
- Explain the concepts of ocean chemistry and ocean acidification.
- Write balanced chemical equations for equilibrium reactions based on experimental observations.
- Explain the interdependency of natural systems.
- Use the scientific method to develop hypotheses based on previous knowledge.
- Communicate scientific information.
- Use Le Chatelier’s principle to predict experimental and theoretical outcomes.
- Apply chemical knowledge and scientific thinking to real-world problems.
- Explain how the addition of carbon dioxide to seawater lowers pH.
- Describe using chemical equations how adding carbon dioxide to seawater impacts ocean chemistry.
- Make predictions and draw conclusions based on data.
Introduction
Before coming to the lab this week, read Chapter 14 on acid-base equilibria and Section 15.2 on solubility products. The information provided in these sections will help you complete the prelab assignment and understand our work this week.
This case study is an adaptation of Equilibria and the Environment by Tracy J. Terry.1
We learned in a case study earlier this semester that when we breathe into water, the pH of the water becomes more acidic. This doesn’t happen when we blow ambient air into water because the concentration of carbon dioxide is approximately 100 times more concentrated in an exhale than in ambient air.2 This means we introduce a lot more carbon dioxide directly into the water sample, shifting the equilibrium of the mixture, which results in a change in pH.
Video 1.0 explains the Carbon Cycle and how carbon dioxide is added to the atmosphere and, eventually, our oceans.3 Please watch this video before coming to lab this week.
Video 1.0 – The Carbon Cycle
The Chemistry of the Ocean
The oceans contain many ions, most abundantly sodium and chlorine. The oceans contain between 33-37g/L (generally noted as 35ppt) dissolved sodium chloride.4 The oceans also contain small amounts of magnesium, calcium, carbonate, bicarbonate, sulfate, nitrate, and trace amounts of gold. The average pH of the oceans was 8.2 before the Industrial Revolution. Today, the average pH has dropped below 8.1.5 This increase is due to the overproduction of carbon dioxide by human activities, impacting the equilibrium of our oceans.
The Importance of Soluble Calcium Carbonate
Calcium carbonate is the major chemical component of shellfish shells. Shellfish include mollusks (i.e. clams, muscles, scallops, and oysters) and crustaceans (i.e. crayfish, shrimp, crab, and lobster).6 Their shells are made using two forms of calcium carbonate: (1) calcite, the most common crystalline form, also found in chalk, marble, coral, and limestone,7 and (2) aragonite, which is less stable than calcite and will form calcite at higher temperatures and pressures.8 Shellfish can repair their shells using the calcium and carbonate ions dissolved in ocean water. However, when the pH of the ocean changes, this impacts the presence of carbonate ions in water.
Today, we will investigate the solubility of calcium carbonate and the impact of pH on its solubility. We will also learn more about the impact of increased atmospheric carbon dioxide on the ocean’s chemistry. You will then have time to work with your lab partner to develop your experimental procedure for the Ocean Acidification Experiment and begin your experiment after we have completed the case study.
You do not need to write anything in your lab notebook for the case study; however, you will still need to bring your lab notebook to class for the Ocean Acidification Experiment we will begin after the case study.
Pre Lab Assignment1
- Write the chemical equation for the dissolution of carbon dioxide in water. Note the phase of matter for each chemical. (4 points)
- Give an example of a Bronstead-Lowry acid and base. (2 points)
- Explain the relationship between pH and hydronium concentration. (2 points)
- Calculate the concentration of hydronium and hydroxide for a solution with a pH of 5.2. (2 points)
- Below is a list of the most common dissolved ions in seawater. For each ion, determine if it creates an acidic, basic, or neutral solution when dissolved in water. Write the balanced equation for its interaction with water. Remember that reactions involving a weak acid or base will exist in an equilibrium. Indicate this equilibrium using a double arrow. If the reaction is one-directional, use a single arrow to represent this. If no reaction occurs, write “NR.” (16 points)
Write the equations for the following ions when added to water:
Sodium Ion
Chlorine Ion
Magnesium Ion
Calcium Ion
Bromine Ion
Carbonate Ion
Bicarbonate Ion
Potassium Ion
Case Study
We will asnwer the following questions during lab time. Please bring either a printed copy of this page to class or a device (such as a laptop or tablet) to access this page. You will also need paper and a pen to take notes/answer the questions provided below.
- What is the formula for calcium carbonate, the major component of seashells?
- Using the Ksp for calcium carbonate found in Appendix J of the textbook, write the balanced chemical equation for its dissolution in water. Based on the Ksp value, indicate if this reaction favors the products of the reactants.
- Write the equation for the dissolution of carbon dioxide in water. Note the phases of matter for each chemical.
- Write the dissociation for carbonic acid in water. Note this is a diprotic acid. Note the phases of matter for each chemical.
- Based on what you understand about the dissociation of acids, do you expect to find more bicarbonate and carbonate in water? Use the Ka values in Appendix H of your textbook to support your conclusions.
- Your instructor has three beakers of aqueous solutions on her desk – one basic, one neutral, and one acidic. Your instructor will tell you the pH of each solution. Write a hypothesis regarding what you think will happen when calcium carbonate is added to each beaker. Once you have written your hypothesis, your instructor will conduct an experiment to test it.
- How did the change in pH impact the dissolution of the calcium carbonate?
- Write the reaction for calcium carbonate and each solution. Indicate the phase of matter of each chemical present. If no reaction occurred, write NR on the product side.
- How do the results of this experiment relate to OA?
References
1 Terry, T. Equilibria and the Environment. National Science Teacher’s Association Case Study Database. February 18, 2020. https://www.nsta.org/ncss-case-study/equilibria-environment (accessed on 2020-12-04.).
2 BYJU’s. Gasses: We Breathe In and Breathe Out. https://byjus.com/biology/composition-gases-breathe/ (accessed 2024, Feb 15).
3 U.S Environmental Protection Agency. The Carbon Cycle. U.S. Environmental Protection Agency. YouTube, Sept 14, 2012. https://youtu.be/vrDekmRbBVk?si=3Z0JJ7xJzeYdfAY- (accessed 2024-2-23)
4 NOAA Sea Water. https://www.noaa.gov/jetstream/ocean/sea-water#:~:text=The%20two%20most%20common%20elements,parts%20per%20thousand%20(ppt). (accessed 2024-2-23)
5 EPA – Understanding the Science of Ocean and Coastal Acdification. https://www.epa.gov/ocean-acidification/understanding-science-ocean-and-coastal-acidification#:~:text=Prior%20to%20the%20Industrial%20Revolution,ten%2Dfold%20increase%20in%20acidity. (accessed 2024-2-23)
6 Horne, F. How are seashells created? Or any other shell, such as a snail’s or a turtle’s? Scientific American. [Online], October 23, 2006. https://www.scientificamerican.com/article/how-are-seashells-created/ (accessed 2024, Feb 15).
7 Wood’s Hole Oceanographic Institution. Know Your Ocean: Did You Know? How are Seashells Made? https://www.whoi.edu/know-your-ocean/did-you-know/how-are-seashells-made/ (accessed 2024, Feb 15).
8 Jones, B. Aragonite. https://www.rockngem.com/aragonite/ (accessed 2024, Feb 15).
This page was published on February 14, 2024 and last updated on February 27, 2025.
©Catherine Haslag 2025. All Rights Reserved.