Objectives
- Conduct laboratory work in compliance with guidelines for personal lab safety and responsible management of chemical waste; this includes appropriate use of personal protective equipment and interpretation of Globally Harmonized System for Hazard Communication (GHS) labels.
- Measure quantities such as mass, volume, temperature, and absorbance with proper technique, and record the results of measurements with the appropriate number of significant figures and units.
- Record observations of chemical processes (such as precipitate formation, gas evolution, etc.) and write chemical reactions consistent with their observations.
- Demonstrate proper techniques for laboratory procedures, such as titration, filtration, solution preparation, spectrophotometric measurements, etc.
- Demonstrate proper use of glassware and equipment including beakers, Erlenmeyer flasks, volumetric pipets, burets, volumetric flasks, watch glasses, graduated cylinders, filtration apparatus, single-beam spectrophotometer, pH meter, balances.
- Communicate lab procedures, observations, and results in the form of laboratory notebook, written reports, and verbal presentations effectively.
- Interpret and analyze qualitative observations and quantitative results, incorporating graphs and tables as appropriate.
- Write balanced chemical equations.
- Calculate Ksp using solubility data.
- Explain titration.
- Experimentally determine the molarity of an unknown solution.
- Explain the purpose of an indicator.
- Perform dilution calculations.
- Read scientific journal articles.
- Identify errors and explain their effect on experimental data.
- Draw conclusions from experimental data.
Introduction
Read the chapter in your textbook on solubility product before beginning this lab. The calculations conducted in the lab are similar to those we learned in the lecture. You may also want to review the lecture videos on determining solubility product to assist you with this lab.
Please review the videos below on using a volumetric pipet and a buret.
Video 1.0 – How to use a volumetric pipet.
Video 2.0 – How to use a buret.
When calcium reacts with water, solid calcium hydroxide forms, as illustrated in Equation 1. Since a small amount of calcium hydroxide is soluble in water, some of those calcium hydroxide particles will dissolve in water (Equation 2).
Ca(s) + 2H2O(l) → Ca(OH)2(s) + H2(g) Equation 1
Ca(OH)2(s) ↔ Ca2+(aq) + 2OH–(aq) Equation 2
In this lab, we will react elemental calcium with water to produce calcium hydroxide. We will filter the solid calcium hydroxide from the solution and then titrate the dissolved calcium hydroxide in solution with a known concentration of hydrochloric acid. Using titrimetric calculations, we will determine the Ca2+ and OH– concentrations in solution. Finally, we will use the solubility product expression for calcium hydroxide to determine the solubility product, Ksp, for the compound.
You will be doing volumetric dilutions and calculating percent error for the Ksp you calculate in the lab. Previous introductions to the labs we have done this semester and your textbook provides these formulas. Reading the article titled Determination of Ksp, ∆G, ∆H, and ∆S for the Dissolution of Calcium Hydroxide in Water and Section 16.4 of the textbook to learn more about the connection between Gibbs Free Energy and Solubility Product. I recommend you find the formulas relating solubility product and Gibbs Free Energy and include them in the introduction to this lab to reference as you conduct the experiment and process your data.
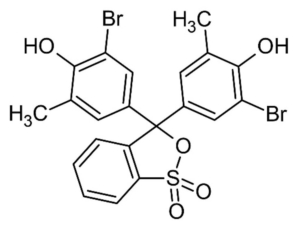
We are using bromocresol purple as the pH indicator for this lab. Figure 1 provides the structure for this compound. Bromocresol purple is yellow at acid pH and purple and basic pH. Since we are titrating the calcium hydroxide with hydrochloric acid, this is a strong acid/base titration, and the reaction’s endpoint will be at pH 7. Since bromocresol purple goes from yellow to brown and then purple between pH of 5.2 and 6.8, this indicator is ideal for determining the reaction’s endpoint. Figure 2.0 illustrates how bromocresol purple changes from yellow to purple as the pH changes.
Figure 1
Structure for Bromocresol Purple
Figure 2
Bromocresol Purple pH Indicator. Bromocresol purple changes its color depending on the pH of the solution. Here bromocresol purple was added to two beakers containing different solutions. Bromocresol purple is yellow below a pH of 5.2 and purple above a pH of 6.8.
Creating a Data Table
You must create a data table(s) for this lab. I recommend setting it up before coming to the lab as it will make your experiment easier and keep your data neatly organized. If you prefer to create your data table as you complete your experiment, you are welcome to do that but remember you need to keep it neat and organized so others can clearly and easily read and follow your data.
When designing your data table, consider what you measure in the lab. You need to create space to record that data in your table. We will also be calculating concentration, dilution, and solubility product (Ksp) in the lab. Create a column in your table that includes this calculated data with the other data you will collect in the lab.
If you would like to see a video on how to create a data table, watch the video to the right.
Video 3.0 – How to make a data table.
Safety Information
The safety data sheets for each chemical we will use in this week’s lab are linked below. I encourage you to review these before coming to lab.
- Calcium is a strong reducing agent. Do not let the calcium touch your skin. Instead, handle the calcium using tweezers. You can also wear gloves if you wish. If you get solid calcium on your skin, wash it immediately with soap and water. Calcium also produces hydrogen gas when it reacts with water. Hydrogen is very flammable. Do not conduct this reaction in the presence of a flame.
- Calcium hydroxide is corrosive and a skin irritant. Do not let it touch your skin or clothes. If you get calcium hydroxide on your skin, wash it immediately with soap and water. If you spill any on your clothes, remove the affected clothing immediately and wash the affected area of your skin with water.
- Hydrochloric Acid is a corrosive acid that can cause damage to eyes and skin. It can also irritate your lungs if inhaled.
- Bromocresol Purple is not a hazardous chemical. Can irritate eyes and skin.
Nitrile gloves and chemical aprons are available for student use in the chemistry lab. Wear your goggles throughout this lab.
Pre Lab Lecture Video
Click on the image to the right to watch the pre-lab lecture video for this experiment. The information in this video will help you come to the lab prepared to complete this experiment.
Video 4.0 – Pre-lab lecture video. Click on the image above to view the video. It will open in a new window.
Experimental Determination of the Solubility Project of Calcium Hydroxide Pre-Lab Assignment
Answer the questions below on a piece of notebook paper before the start of the lab. They are due at the teacher’s desk before the beginning of the lab. Failure to complete these questions before the start of the lab will result in a zero for this experiment. (10 points)
- What is the indicator we are using in this lab? How will you know when you have titrated your reaction to the endpoint? (2 points)
- Write the solubility product expression for calcium hydroxide below. Also record this in the introduction in your lab notebook. (2 points)
- Write the balanced chemical equation for the reaction of calcium hydroxide with the acid we are using in this lab. Also record this in the introduction in your lab notebook. (2 points)
- Using Appendix J of your textbook, write is the known (theoretical) solubility product (Ksp) for calcium hydroxide below. Also record this in the introduction in your lab notebook. (1 point)
- Using the Ksp value obtained from Appendix J, calculate the concentration of hydroxide ions present in a saturated solution of calcium hydroxide. Include this value in the introduction of your lab notebook. You will need this for the lab. (3 points)
Experimental Supplies
Calcium, solid
2.000M Hydrochloric Acid
Bromocresol Purple
250mL Erlenmeyer flasks
Stir plats and stir bar
1mL pipet and bulb
25mL pipet and bulb
250mL beaker
100mL volumetric flask
Burette
Burette Clamp
Burette Stand
100mL Graduated Cylinder
Thermometer
Filter paper
Stoppers
Tweezers
Glass Funnel
DI Water
BOILED DI Water
Paper Towels
Experimental Procedure
Experimental Supplies
1. Using a pair of tweezers, obtain a small piece of solid calcium from the chemical jar and add it to a beaker containing approximately 150mL of BOILED DI water. (DO NOT use regular DI water from the tap for this step.) Do not add too large an amount as this will create a lot of precipitate, which could be hard to filter from the solution. Be careful to add enough calcium so that you saturate the solution. You should see a small amount of white calcium hydroxide form in the solution. Stir the beaker and allow the precipitate to settle.
2. Using gravity filtration, remove the precipitate from the solution. DO NOT WASH THE PRECIPITATE.
3. Once the filtration is complete, stopper the solution to prevent the solution from reacting with the carbon dioxide in the air to form calcium carbonate. This side reaction will impact your experimental results. Your solution should be completely clear. If there is any precipitate present, filter the solution again.
4. Record the exact concentration of the HCl solution in your lab notebook. Next, you need to create a dilute solution of the HCl by volumetric dilution of 1:100 (i.e., 1mL of HCl to 100mL of solution). Finally, determine the new concentration of your HCl solution to 3 sig figs.
5. Prepare the burette with the HCl solution you created as we did in the Acid-Base Titration lab, thoroughly rinsing the burette with DI water and then a small aliquot of HCl.
6. Fill the burette with the HCl solution you created. Record the starting volume of HCl on the table in your lab notebook.
NOTE: The next steps of your titration need to occur quickly to minimize the reaction of carbon dioxide from the air with the saturated calcium hydroxide solution. Stopper the Erlenmeyer when you are not immediately adding something to it. Complete the next 3 steps as quickly as possible.
7. Pipette 25mL of the calcium hydroxide solution into a 250mL Erlenmeyer. If there is scum on the solution’s surface, do not pick any of it up with the pipet. Record the volume of the calcium hydroxide solution in your lab notebook. Add 3-4 drops of bromocresol purple indicator and an additional 50mL of BOILED DI water to the Erlenmeyer.
8. Place a stir bar in the Erlenmeyer and set the stir plate to mix the solution quickly without splashing. Your solution should appear purple when thoroughly mixed. DO NOT TURN ON THE HEAT.
NOTE: Prepare your calcium hydroxide solutions immediately before you are ready to titrate them. Keep the Erlenmeyers stoppered at all times to prevent the solution from reacting with the carbon dioxide in the air.
9. Titrate the calcium hydroxide solution to the endpoint (until it just turns brown/yellow) using the diluted HCl solution. Record all volumes of HCl to 0.01mL in your lab notebook.
10. You will repeat steps 6-8 until you have performed a total of four titrations of your calcium hydroxide solution.
11. Take the temperature of one of your trials. Since the solutions have been sitting in the same space for an extended time, they will all be the same temperature (around room temperature).
Waste Disposal
- Wash all of the solutions down the drain with lots of water.
- Place the filter paper and paper towels in the trash.
- Wash the burette, rinsing with DI water, and place it tip-up in the clamp. Place the burette, clamp, and stand by the drying oven.
- Place the 100mL volumetric flask and 250mL Erlenmeyer flasks in the tub on your teacher’s desk.
- Wash any other equipment used with soap and DI water (the final rinse with DI water) before putting it away.
Assignment
Once you have completed this experiment and recorded all of your data in your laboratory notebook:
- Write the discussion and conclusions sections for the lab. The questions in the post-lab assignment will help you process the data from this lab. Be sure to include all of the calculations from the experiment in your lab manual.
- Calculate the [OH–] for each of the trials.
- Calculate the Ksp for calcium hydroxide for each trial.
- Using Excel, determine the average Ksp for the four trials and their standard deviation. Include this information in your lab manual.
- Calculate the percent error for your experimentally determined Ksp for calcium hydroxide based on the known (theoretical) you found in Appendix J.
- Complete the post-lab assignment and submit it to your instructor.
- Enter your average Ksp value into the classroom data spreadsheet.
- Answer the post-lab questions below on a piece of notebook paper. These are due at the start of the next lab period. Reading the article titled Determination of Ksp, ∆G, ∆H, and ∆S for the Dissolution of Calcium Hydroxide in Water and Section 16.4 of the textbook to learn more about the connection between Gibbs Free Energy and Solubility Product. This will help you with the post-lab assignment.
- I provided some guidance in the prelab video regarding calculating the Ksp for calcium hydroxide.
Post-Lab Questions
Using the information you gathered during today’s lab, answer the following questions. This assignment is due at the start of the following lab period. (15 points)
- How would your results be impacted if there was still solid calcium hydroxide in the solution when you titrated? Specifically, explain how this would affect your experimentally determined solubility product. (2 points)
- Show your calculations for determining the [OH–] and [Ca2+] and finding the experimental Ksp for Trial 2 in the space below. (2 points)
- Write your experimental values for your Ksp for all four trials below. Calculate the average Ksp for calcium hydroxide. (2 points)
- Determine the percent error for your experimental Ksp Show your calculations. (2 points)
- Using the Ksp you determined experimentally, calculate the Gibbs free energy for each trial you performed. The scientific article provided discussed how Gibbs Free Energy applies in this experiment. What does your calculated Gibbs Free Energy value tell you about the reaction? (3 points)
- Determine the ∆G° for the reaction of Ca metal with water using the values from Appendix G in your textbook. This will be the theoretical value for the ∆G° of this reaction. Comment on the accuracy and precision of the ∆G° values you determined using your experimental data. (4 points)
Formal Lab Report
You must write a formal lab report on the experiment and your results. Please see the Guide for Writing a Formal Lab Report provided in the General Course Materials section of Brightspace. The due date for this assignment is noted on the semester schedule.
Below is some experiment-specific information you will need to include in your report:
- A data table outlining your data and results.
- Calculate the [OH–] and [Ca2+] for each trial.
- Determine the Ksp for calcium hydroxide for each of the four trials. Using Excel, determine the average Ksp for the four trials and their standard deviation.
- Calculate the percent error for your experimentally determined Ksp for calcium hydroxide based on the known (theoretical) you found in Appendix J.
- Show one sample calculation to determine the [OH–] and [Ca2+] and find the experimental Ksp.
- Use Determination of Ksp, ∆G°, ∆H°, and ∆S° for the Dissolution of Calcium Hydroxide in Water article in your introduction to discuss solubility product and how different factors impact the Ksp of calcium hydroxide.
- Report the mean, median, range, and standard deviation for the classroom data provided in this spreadsheet.
A rubric for this formal lab report can be found on Brightspace if you go to Assignment → Rubrics → Experimental Determination of the Solubility Product of Calcium Hydroxide Formal Lab Report Rubric
References
Euler, Kirschenbaum, L. J., & Ruekberg, B. (2000). Determination of Ksp, ΔG0, ΔH0, and ΔS0. Journal of Chemical Education, 77(8), 1039–. https://doi.org/10.1021/ed077p1039
Fisher Scientific. (2014). Safety Data Sheet – Calcium Metal. https://www.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-c/S25217.pdf
Fisher Scientific. (2014). Safety Data Sheet – Calcium Hydroxide. https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-c/S25225.pdf
Thermo Fisher Scientific. (2021). Safety Data Sheet – Bromocresol Purple. https://www.fishersci.com/store/msds?partNumber=AC151330050&productDescription=BROMOCRESOL+PURPLE%2C+INDI+5GR&vendorId=VN00032119&countryCode=US&language=en
ScienceGonnaGetYou. (2015, October 14). How to Make a Data Table [Video]. YouTube. https://youtu.be/vatJYQEV-qA
Sigma Aldrich. “calcium.” Datasheet, Sep 2024. https://www.sigmaaldrich.com/US/en/sds/aldrich/441872?srsltid=AfmBOopugJcOVbLd9CbM60NzosWBepWNqM7iOxuOgITLGMrgDa_Fda97
Thermo Fisher Scientific. “bromocresol purple.” Datasheet, Oct. 2010. [Revised Dec. 2021]. https://www.geneseo.edu/sites/default/files/2022-10/BROMOCRESOL-PURPLE%20sds.pdf
Thermo Fisher Scientific. “calcium hydroxide.” Datasheet, Oct. 2010. [Revised Oct. 2023]. https://www.fishersci.com/store/msds?partNumber=C973&productDescription=CALCIUM+HYDROXIDE+CERTFD+3KG&vendorId=VN00033897&countryCode=US&language=en
Thermo Fisher Scientific. “hydrochloric acid.” Datasheet, Aug. 2009. [Revised Oct. 2023]. https://www.fishersci.com/store/msds?partNumber=A481212&productDescription=HYDROCHLORIC+ACID+NF%2FFCC+21%2F2L&vendorId=VN00033897&countryCode=US&language=en
University of California, Santa Cruz. (2011). Lab 10 – Solubility Product for Calcium Hydroxide. General Chemistry, First Edition. https://www.webassign.net/labsgraceperiod/ucscgencheml1/lab_10/manual.html
This page was published on March 28, 2023 and last updated on April 14, 2025.
©Catherine Haslag 2025. All Rights Reserved.