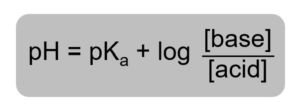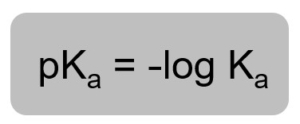Objectives
- Conduct laboratory work in compliance with guidelines for personal lab safety and responsible management of chemical waste; this includes appropriate use of personal protective equipment and interpretation of Globally Harmonized System for Hazard Communication (GHS) labels.
- Measure quantities such as mass, volume, temperature, and absorbance with proper technique, and record the results of measurements with the appropriate number of significant figures and units.
- Record observations of chemical processes (such as precipitate formation, gas evolution, etc.) and write chemical reactions consistent with their observations.
- Demonstrate proper techniques for laboratory procedures, such as titration, filtration, solution preparation, spectrophotometric measurements, etc.
- Demonstrate proper use of glassware and equipment, including beakers, Erlenmeyer flasks, volumetric pipets, burets, volumetric flasks, watch glasses, graduated cylinders, filtration apparatus, single-beam spectrophotometer, pH meter, balances.
- Communicate lab procedures, observations, and results in a laboratory notebook, written reports, and verbal presentations effectively.
- Interpret and analyze qualitative observations and quantitative results, incorporating graphs and tables as appropriate.
- Identify errors and explain their effect on experimental data.
- Design an experimental procedure.
- Apply knowledge of scientific theories to problem-solving applications.
- Develop a hypothesis for a scientific experiment.
- Identify the control, independent, and dependent variables for an experiment.
- Predict the next steps for a scientific study using collected data.
- Draw conclusions based on experimental data.
- Discuss the results of an experiment in oral and written formats, including predictions, graphs, and calculations.
- Research and complete an analysis of scientific findings on a current topic in chemistry.
Introduction
Please read section 14.6 of your textbook on buffers, paying special attention to the information Henderson-Hasselbalch equation. The Henderson-Hasslebalch equation relates pH of a buffer to the concentration of the conjugate acid-base.
Figure 1: Henderson-Hasslebalch Equation
You will need the pKa value for the conjugate acid used. You can find this using the question below:
Figure 2: Formula for determining pKa from Ka
While we will use buffer tablets to create our buffers this week, you can make your own buffers using the Henderson-Hasslebalch equation and the Ka values for weak acids.
Appendix H of your textbook lists of Ka values for most the weak acids listed below. You may also need to look up some of the Ka values online. This resource can help identify the chemicals you need to prepare a buffer solution. Then use the Henderson-Hasslebalch equation to determine how much of the conjugate acid-base pair you will need to create your buffer.
The pKa of each conjugate acid can be used to identify the best conjugate acid-base pair needed to make a buffer. For instance, if you want to create a buffer with a pH of 4, then choose a conjugate acid with a pKa close to 4 to make your buffer.
Below is additional reference material that may help you create buffer solutions for this lab.
Buffer Reference Center created by Millipore Sigma
This week, you will test the impact of increasing acidity on calcium carbonate shells. You will write your experimental procedure using the guidance provided in this lab. You will then set up your experiment and monitor it for the next 3 weeks (counting the first week of lab, this is 4 weeks total). Finally, you will report on your findings in a lab report. This entire process is designed to demonstrate how scientists learn about a process, develop experiments to learn more about/test what is occurring, and then share their data with other scientists.
Experimental Procedure
Experimental Materials
pH 6, 7, 8 and 9 buffer capsules
Four 150mL beakers (take these from the cabinets in the classroom)
DI Water
Glassware in your drawer
Vernier Interface and pH probe
Digital Balance
4 Sea Shells
Plastic Pipets
Label Tape
Sharpe Marker
Paper Towels
Experimental Purpose
The purpose of the lab is to design an experiment to investigate the impact of increasing acidity on sea shells over time.
Experiment Outline
Design a lab to test the effect of pH on sea shells. Below are some parameters for your experiment.
- Design and write a step-by-step experimental procedure. You will be provided the experimental supplies listed above. Your procedure should include all steps of your experiment, including directions on making any necessary solutions. (Hint: check the side of the buffer containers for instructions on how to use the capsules.)
- You will create four aqueous buffer solutions using the chemicals provided by your instructor. Record the pH of each solution at the start and each time you take the mass of the sea shells.
- This experiment will last for 4 Mondays. This week is the first Monday. Each Monday, you will measure the mass of your sea shells and record the data in your laboratory notebook. You will collect a total of 4 data points during this experiment.
NOTE: You may not need to use all of these supplies. That is okay. You may find you need additional supplies to complete the experiment. If you need additional supplies beyond this list, please talk with your instructor to obtain them.
You will not be given any chemicals beyond those in the list above for the experimental materials.
Before leaving lab today, you need to:
- Write your hypothesis for this experiment.
- Identify the control, dependent, and independent variables.
- Identify the control sample.
- What qualitative and quantitative data are you collecting in this lab? (Hint, you should have both forms of data.) List the data you will collect at the start, during, and at the end of the lab.
- A list of the specific materials you need for the experiment.
- Write your experimental procedure. Please type your experimental procedure. When your experimental procedure is drafted, have your instructor review it for feedback.
- Email a copy to your instructor and lab partner after your procedure is approved by your instructor. Your instructor will print copies for you to paste into you lab notebook (glue sticks available on your instructor’s desk).
- You can begin your experiment once your experimental procedure is approved and glued into your lab notebook.
Before Next Week’s Lab:
- Copy/paste your experimental procedure into your lab notebook.
- Draw a table in your experimental notebook to collect your data.
- Write down the buffer solution information and any relevant safety information for this lab. This includes the pH of each solution and the chemicals (name and formula) used to create each buffer. You can find this information in the SDS sheets linked below:
Leave a few pages blank at the end of this lab to allow you to collect your data and write your discussion and conclusions section. We will conduct this experiment while we continue with other experiments in the class. If you don’t use all the blank pages by the time the experiment is complete, draw a line through the page and initial and date the page indicating you meant to leave the page blank.
Post Lab Assignment
- Graph your lab data for each of your samples. You want to graph your dependent variable vs. time for each sample. Your graph should have a total of 4 lines (one for each pH solution). Include the R2 value and equation for each line. Title your graph and label the axes and samples. Paste this in your lab notebook.
- Write up your discussion and conclusion in your lab notebook. Be sure to note if you reject or accept your hypothesis and why.
Ensure you have experimental information and data in your lab notebook. You will write a lab report on your experiment and findings. The better organized and complete your procedure and data, the easier it will be to write your report.
You do not need to submit this to your instructor; however, they are happy to look your information over before you write you lab report to ensure you have a complete graph and have interpreted your data correctly.
Lab Report
You must write a lab report on the experiment and your results. Please see the guidelines on How to Write a Lab Report. The due date for this assignment is noted on the semester schedule.
Below is some experiment-specific information you will need to include in your report:
- The experimental procedure and a complete list of experimental supplies. Be sure to include any updates you made to your procedure while you conducted your experiment. Since you wrote this portion of your experiment, this section will be worth more points than on previous lab reports. Take the extra time to ensure its complete and clear.
- A data table(s) outlining your results/data. Basically, if you recorded a piece of quantitative or qualitative data during this experiment, it should be in this table. If it makes more sense for you to include two or more tables to summarize your data, that is fine too.
- Include the graph you created using your data (percent change in mass vs time). Your graph should include the equation and the R2 value for each line.
- Discuss the accuracy and precision of the data points you collected. If you chose to throw out a data point, explain why.
- What do your results tell you about ocean acidification’s impact on animals with shells?
- The introduction of your lab report should include a summary of ocean acidification and how it occurs. You should cite at least one source for this information. You can use any of the articles, videos, or other resources we have used in this class regarding OA as your source or you can find a different source to use. This source and any other sources you use would be cited at the end of your lab.
- Don’t forget to properly format subscripts, even on your data tables/graphs.
- You will work with your partner to write your lab report. Include ALL of your names on the lab report (you are all contributing scientists to this research). Everyone in each group is expected to work cooperatively and equally with each other. If your partner isn’t contributing their fair share, please come talk to me about the situation.
- Post (i.e. publish) your lab report to the Ocean Acidification Lab Report discussion board on Brightspace. This allows the class to read your research. This is like submitting your paper to a journal or other forum to share with other scientists.
Remember that this is your research. You are the expert on what you did in the lab. Write this report as if you are reporting your results to other scientists who don’t know much about ocean acidification.
A rubric for this formal lab report can be found on Brightspace if you go to Assignment → Rubrics → Ocean Acidification Lab Report Rubric.
References
This page was published on February 1, 2024 and last updated on March 19, 2025.
©Catherine Haslag 2025. All Rights Reserved.