Objectives
- Conduct laboratory work in compliance with guidelines for personal lab safety and responsible management of chemical waste; this includes appropriate use of personal protective equipment and interpretation of Globally Harmonized System for Hazard Communication (GHS) labels.
- Measure quantities such as mass, volume, temperature, and absorbance with proper technique, and record the results of measurements with the appropriate number of significant figures and units.
- Record observations of chemical processes (such as precipitate formation, gas evolution, etc.) and write chemical reactions consistent with their observations.
- Demonstrate proper techniques for laboratory procedures, such as titration, filtration, solution preparation, spectrophotometric measurements, etc.
- Demonstrate proper use of glassware and equipment, including beakers, Erlenmeyer flasks, volumetric pipets, burets, volumetric flasks, watch glasses, graduated cylinders, filtration apparatus, single-beam spectrophotometer, pH meter, balances.
- Communicate lab procedures, observations, and results in a laboratory notebook, written reports, and verbal presentations effectively.
- Interpret and analyze qualitative observations and quantitative results, incorporating graphs and tables as appropriate.
- Predict the products of a chemical reaction.
- Predict the formation of a precipitate in aqueous solution.
- Determine if a salt will form an acidic or basic aqueous solution.
- Create a flow chart to experimentally determine the identity of unknown ions in aqueous solution.
Introduction
Qualitative analysis is used to determine the presence or absence of an ion/compound. In qualitative analysis, we are not concerned with the amount of the ion/compound present as we are in quantitative analysis. We are only concerned with an ion/compound’s presence or absence.
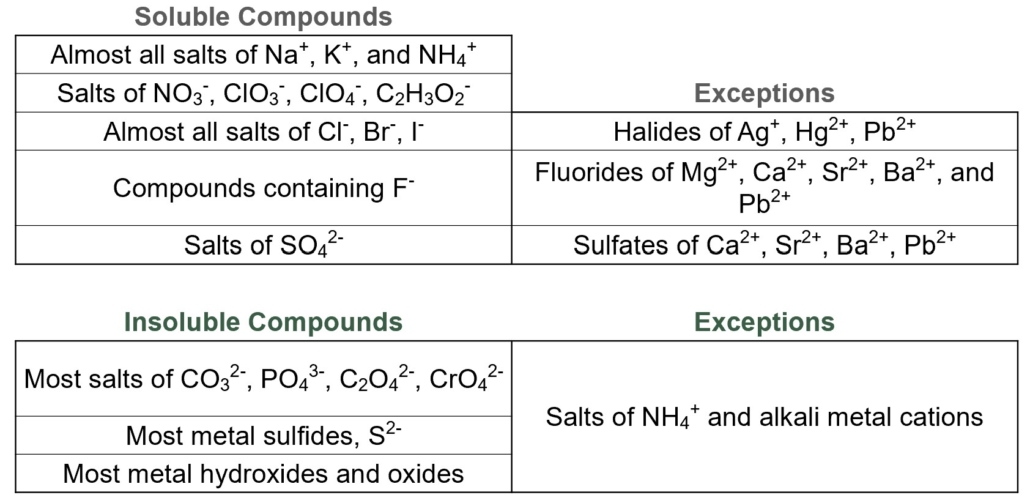
To complete this analysis, we will use our understanding of acid-base chemistry, chemical reactions, and solubility to conduct these tests. Figure 1.0 summarizes the solubility rules we learned in General Chemistry 1.
If you need a refresher of any of these concepts, please refer to the following sections of the textbook:
Section 4.1 discusses writing and balancing chemical equations
Section 4.2 discusses types of chemical reactions, including precipitate reactions
Figure 1.0 – Solubility Rules
Testing Anions and Cations
In this week’s lab, you will be provided with 2 unknown solid compounds. You and your partner need to determine the cation and anion present in each compound. Correctly identifying these two compounds is worth 10 points of the week’s grade (5 points per compound). Before beginning your testing, dissolve a pea-sized amount of the compound in 10mL of water in a large test tube. This solution will be enough to conduct all of your tests. Below are lists of possible cations and anions you will need to test for in this week’s lab. These will be provided to you in the list of chemicals called Standard Solutions in the experimental materials.
Cations: Na+, Li+, K+, Ca2+, Mg2+, Sr2+, Ba2+, Ag+, and Pb2+
Anion: chloride, hydroxide, sulfate, carbonate, bicarbonate, acetate, and nitrate
While at first glance it appears there are 63 possible compounds for this week’s lab, remember that not all of these 63 compounds are soluble in water. Keep this information in mind as you conduct this week’s experiment. It will help you narrow down the identity of your cation and anion.
You need to create a flow chart to help you determine the identity of the cation and anion for each of your unknowns. Using your understanding of chemical reactions, acid-base chemistry, and solubility rules will help you do most of this work (even before you come to class). You will also have test solutions for each of the ions available so you can perform a test with the known compound and compare the results to your unknown.
You will also need a confirmation test for each of your ions (except for acetate and nitrate). For most of the cations, this will be the flame test. Your confirmation test for the anions will vary. A confirmation test is a definitive test performed after you think you know the identity of the ion. This is a test that will definitively confirm the ion’s identity. For example, if you think copper is present in your sample, the presence of a green flame during the flame test would confirm this.
NOTE: You may not need to complete all of the tests I provide here to determine the identity of your unknown. This is ok.
The experimental procedure outlines the equipment and chemicals available for this lab and the tests you will perform to determine the identity of your unknowns.
Recording Your Data
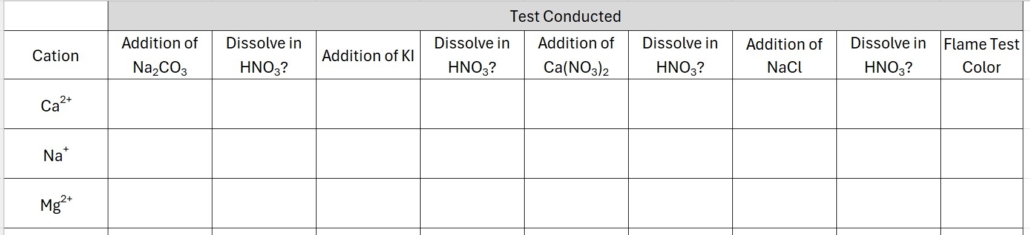
You will need to create data tables to record your data. I recommend one for cations and one for anions. List the ion down the left side of the table and the test name across the top of the table. Leave enough space for each test to record your results. If no reaction is observed, simply write NR in the data table. Be careful to note the color of any precipitates and if a gas is formed.
You can draw your data table directly into your lab notebook OR you can create it using Excel or Word and paste it into your notebook before lab begins. An example data table for this class is provided in Figure 2.0.
Figure 2.0 – Example Data Table
PLEASE NOTE: This is an example table of how to organize your data and may not fully represent the cations, anions, and tests we will conduct in this experiment. Please see the complete list of ions we are testing in the introduction and outline of each test in the experimental procedure.
Organizing Yourself
This lab has a lot of moving parts. You are using knowledge from earlier in this class and data you collect in lab to complete this experiment. It’s important to be organized before coming to lab. Be sure you have drawn your data tables before you come to class and filled in any data you can from the prelab. For example, you can determine IF a precipitate forms based on the solubility rules, but you don’t know the color of the precipitate or if it will dissolve in acid until you do that part of the experiment in the lab. You then only need to do the reactions you know will produce a precipiate so you can determine the color of the precipitate.
Below is a breakdown of how to approach this lab.
Step 1: Collect All Your Data
Fill in your data tables with as much information as possible before class. Note if a precipitate will form for a reaction or not. Indicate if the salt for the anion will be acidic, basic, or neutral. This will narrow down the number of tests you need to conduct when you get to lab to finish out your data table. If you have already predicted that a precipitate won’t form for a particular ion, there is no need to conduct that test.
A list of all of the tests you will use is provided in the experimental procedure.
Step 2: Draw a Flow Chart
Once you have collected all of the data for your cations and anions, you need to draw a flow chart. The idea behind the flow chart is to organize your data table into an easy-to-follow process for identifying your unknowns. You will do this in lab. The prelab lecture video linked below provides a demonstration of how to use a data table to create a flow chart. I recommend you start with the flow chart for your cations and identify those first. Then you can draw the flow chart(s) to identify your anions. Remember that the unknowns will all be soluble. Some cation/anion combinations cannot happen as a result. (HINT: The solubility rules can help you here).
Step 3: Identify the Unknowns
Once your cation flow chart is ready, identify the cations in the two unknowns your instructor provided. Once you know the cation, you can narrow down the possible anions that could be in your sample (remember that not all cation/anion combinations will be soluble). Once you have narrowed this down, you can analyze your samples to determine the identity of the anions.
Safety Information
We are working with a lot of chemicals in this lab. To review their Safety Data Sheets, search the name of the compound followed by the letters “SDS” using your favorite internet search engine. If you are unable to find a safety data sheet for any of the chemicals in this lab, see your instructor for help.
Nitrile gloves and chemical aprons are available for student use in the chemistry lab. Wear your goggles throughout this lab.
Pre Lab Lecture Video
To the right is the pre-lab lecture video for this experiment. Please click on the image to watch the video. This video will open in Media Space in a new browser window
If you are interested in checking out Car Talk, click here. I highly recommend the credits at the end for a good laugh. Not sure what I am talking about? Watch the prelab video. 🙂
Video 1.0 – Pre-lab lecture video. Click on the image above to view the video. It will open in a new window.
Pre Lab Assignment
Prelab questions (Worth 15 points)
Answer the questions below before the start of the lab. You will complete this prelab work in your lab notebook. We don’t normally do this for lab; however, you will need this background information for the experiment.
Once you have answered these questions, use this information to take the Qualitative Analysis Prelab Quiz on Brightspace. You can check your answers after taking the quiz by reviewing the feedback provided. Please update any of the answers in your lab notebook so you come to lab with all of the correct information to start this lab. Your instructor will walk around and check your notebook to ensure you have this information organized and completed for lab.
1. Using the solubility rules, predict which ions we are testing will precipitate when mixed with each test reactant (see below for the list). The list of ions we are testing is provided in the introduction to this experiment. If a precipitate will form, write the chemical equation for the reaction of the cion with the compound, identifying all phases of matter for the reactants and products.
- You will perform precipitate reactions for the cations using only the following reactants: sodium carbonate, potassium iodide, sodium sulfate, and potassium chromate.
- You will perform precipitate reactions for the anions using only the following reactants: lead (II) nitrate, copper (II) sulfate, and barium chloride.
2. Predict which anions will form basic or acidic aqueous solutions. The list of anions we are testing is provided in the introduction to this experiment.
You cannot complete this lab unless you have completed this prelab assignment (including the quiz on Brightspace) AND record this information in your lab notebook (in the data table). The goal is to know which compounds will perform precipitates and which salts will be acidic, basic, and neutral before you come to class. This will save you some time in the lab.
You will able to review your questions, see what you missed, and get the correct answers to all of these questions by reviewing your quiz and the screen that appears once you submit your quiz.
Please let me know if you have any questions.
Experimental Procedure
Standard Solutions
(The concentration of all standard solutions is between 0.5M and 1M)
The following anions will be provided as a salt formed with sodium or potassium: chloride, hydroxide, sulfate, carbonate, bicarbonate, acetate, and nitrate.
The following cations will be provided as a salt formed with nitrate: Na+, Li+, K+, Ca2+, Mg2+, Sr2+, Ba2+, Ag+, and Pb2+.
Reactants for Tests
1M Sodium Carbonate
1M Potassium Iodide
1M Sodium Sulfate
1M Potassium Chromate
0.5M Lead (II) Nitrate
1M Copper (II) Sulfate
1M Barium Chloride
0.5M Nitric Acid
1M Acetic Acid
Phenolphathalien
Equipment
Red and Blue Litmus Paper
Test Tubes
Disposable Plastic Pipets
Well plate
Glassware in your drawer
Wooden Splint
Spatula/Scoopula
Glass Stir Rod
Striker or Matches
Bunsen Burner
Label Tape
Sharpe
Before beginning any tests, wash your plastic well plate thoroughly with soap and water and rinse with DI water. Place your well-plate face-down on a paper towel and allow to dry for a few minutes. This will ensure that your well plate is clean and prevent contamination from previous use.
Each well on the well plate is noted with a letter and number (see Figure 2.0). You can use this information to track with reaction is in which well during your lab.
Figure 2.0 – Well plate with letter and number well identifier (circled in red).
Tests to Conduct
You can perform the litmus test, the precipitation reaction test, and the dissolution of precipitate using acid test in a well plate. Once you have created an aqueous solution of your unknown compound (dissolve a pea-sized amount of the compound in 10mL of water in a large test tube), place 5-7 drops of the solution with your unknown compound in the well and add 3-5 drops of your reactant test solution to the same well. Record what you observe in your lab notebook.
Litmus Test
Litmus paper turns blue when dipped in a base, red when dipped in an acid, and purple/violet when dipped in a neutral solution. Some of the anions we are testing this week are conjugates to weak acids/bases. This is a quick way to determine if you have one of these conjugate acid/base ions in solution and narrow down the identity of possible compounds.
You already made the predictions for this test in your prelab and wrote this information in your lab notebook. You can use this information for comparison for this test.
You will perform this test for only the anions.
Formation of a Precipitate Test
Precipitation reactions are useful in determining the presence of many ions in solution. Using the solubility rules, you can predict which ions will precipitate when the different reactant test solutions are added to an unknown solution. Be sure to note if a precipitate forms and the color of the precipitate in your observations.
You will have already predicted which combination of cations and anions with the test chemicals will produce a precipitate in the prelab. Perform each reaction that will produce a precipitate to determine the color of the precipitate that forms. Record your observations in your lab notebook.
You will perform this test for all cations and anions as outlined below:
Cation Precipitate Reactant Solutions
You will perform precipitate reactions for the cations using only the following reactants:
- sodium carbonate
- potassium iodide
- sodium sulfate
- potassium chromate.
Anion Precipitate Reactant Solutions
You will perform precipitate reactions for the anions using only the following reactants:
- lead (II) nitrate
- copper (II) sulfate
- barium chloride
Dissolution of Precipitate Using Acid Test
Since some of the anions are conjugates of weak acids, they have basic properties. When these anions form an insoluble salt (precipitate), the precipitate can be dissolved by adding a strong acid to the solution. You will be provided nitric acid to test the solubility of these precipitates.
After you perform the Precipitation Reactions test, add 5-7 drops of nitric acid to the same well as the precipitate and mix with a glass rod. Record your observations in your lab notebook.
You will perform this test for all cations and anions and form a precipitate during the Formation of a Precipitate Test.
Gas Evolving and Neutralization Reactions Test
Some compounds produce a gas when reacted with an acid. Carbonate and bicarbonate are two examples. When reacted with an acid, both will form carbonic acid, a very unstable compound that quickly decomposes into carbon dioxide gas and water. An example of this reaction is provided below. This is a two-step reaction. First the carbonic acid forms, then it decomposes into water and carbon dioxide.
Reaction 1: Na2CO3(aq) + 2HCl(aq) → 2NaCl(aq) + H2CO3(aq)
Reaction 2: H2CO3(aq) → H2O(l) + CO2(g)
Overall Reaction: Na2CO3(aq) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
To determine if your known will form a gas when reacted with acid, place 10 drops of the unknown solution in a well plus two drops of phenolphethalien (to observe any possible pH change due to a neutralization reaction). Add approximately 10 drops of acetic acid quickly. Record your observations in your lab notebook.
You will perform this test only for the anions.
Flame Test
Many of the cations we will test (Ca, Na, Mg, K, Li, Sr, Ba) can be confirmed using a flame test. To conduct this, place a wooden splint in a test tube of the unknown compound dissolved in water. Allow the compound to soak into the wood for 5 minutes. Place the solution-soaked tip of the wooden splint into the hottest part of the flame (the light-blue inner cone) and note the color of the flame that appears.
Known solutions for each of the cations listed above will be provided to you so you can test each and determine the color that appears during the flame test.
You will perform this test for only the cations, excluding Pb and Ag. If you think you have lead or silver ion in your unknown, DO NOT perform a flame test on your sample.
When You Have Idenitied the Cation and Anion For Both of Unknowns
When you have identified the cation and anion in both unknown solids, tell these identities to your instructor. They will confirm the identity of your unknowns and score your results for this part of the lab.
You will know your final score for this lab before leaving class. There is no postlab assignment for this experiment.
Waste Disposal
- Return your unused solid unknown to your instructor.
- If your unknown compound DOESN’T have silver or lead, then the solution you made of your unknown can flushed down the drain with lots of water.
- If you unknown compound DOES contain silver or lead, then give the test tube with your unknown solution to your instructor for proper disposal.
- The used wooden splints can go in the trash.
- Turn your well plate over on a paper towel, allowing the contents to drain onto the towel. Put the towel in the trash and wash your well plate with soap and water. Wear gloves when cleaning your well plate.
- Wash any other equipment used with soap and DI water (the final rinse being with DI water) before putting it away.
References
This page was published on April 8, 2025 and last updated on April 14, 2025.
©Catherine Haslag 2025. All Rights Reserved.