Objectives
- Explain the principles and use of titration in laboratory analysis.
- Determine the amount of solute in a solution.
- Convert between metric units.
- Draw conclusions based on experimental data.
- Identify sources of error in completing laboratory experiments.
- Explain the purpose of an indicator.
- Conduct laboratory work in compliance with guidelines for personal lab safety and responsible management of chemical waste; this includes appropriate use of personal protective equipment and interpretation of Globally Harmonized System for Hazard Communication (GHS) labels.
- Measure quantities such as mass, volume, temperature, and absorbance with proper technique, and record the results of measurements with the appropriate number of significant figures and units.
- Record observations of chemical processes (such as precipitate formation, gas evolution, etc.) and write chemical reactions consistent with their observations.
- Demonstrate proper techniques for laboratory procedures, such as titration, filtration, solution preparation, spectrophotometric measurements, etc.
- Demonstrate proper use of glassware and equipment, including beakers, Erlenmeyer flasks, volumetric pipets, burets, volumetric flasks, watch glasses, graduated cylinders, filtration apparatus, single-beam spectrophotometer, pH meter, and balances.
- Communicate lab procedures, observations, and results in a laboratory notebook, written reports, and verbal presentations effectively.
- Interpret and analyze qualitative observations and quantitative results, incorporating graphs and tables as appropriate.
Introduction
Titration is a quantitative method for the determination of the concentration of an unknown solution using a solution of known concentration, or a standard solution. Titration is only effective for reactions that react completely, or have a 100% yield. Titrations can be classified by the type of reaction that occurs. Examples of titration reactions include acid-base titrations, redox titrations, combination reaction titrations, and back titrations. We conducted acid-base titrations in this class previously. This experiment is a back titration involving a redox reaction.
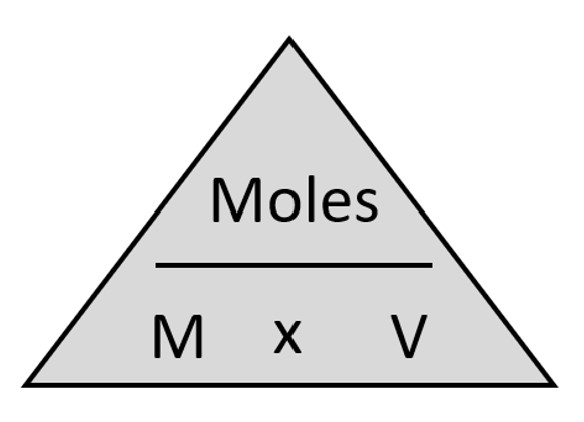
In titration, the reaction is complete when the reaction has reached its endpoint. At the titration endpoint, the quantity of reactant in the titrant added during the titration is stoichiometrically equivalent to the quantity of reactant in the analyte. In other words, enough of each chemical involved in the titration is present so that both chemicals are completely reacted and neither is present in excess. The concentration of the unknown solution can then be determined using the chemical equation for the neutralization reaction and molarity and volume of the standard solution used during the experiment. Figure 1 illustrates the formula for molarity.
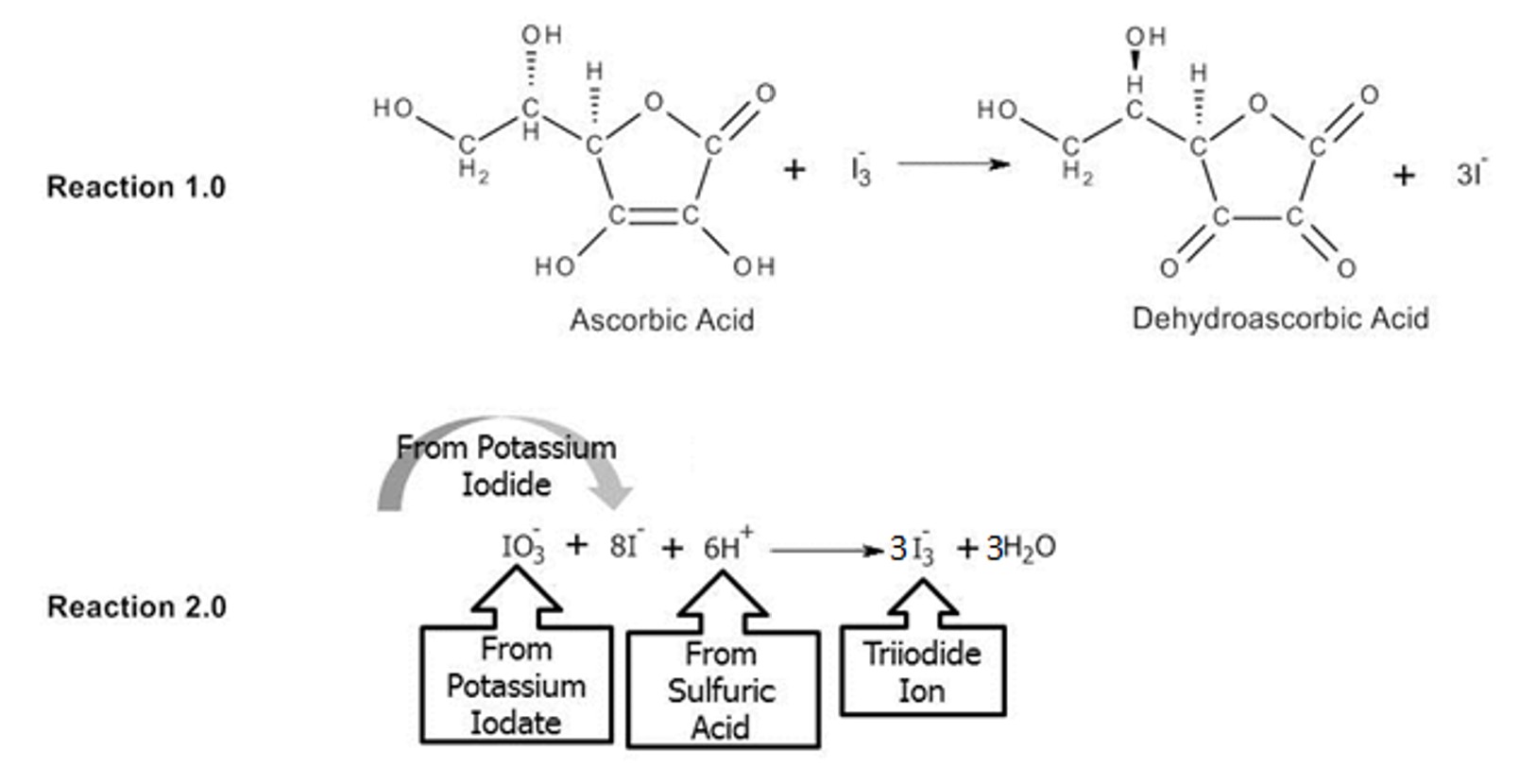
In this experiment, ascorbic acid will be oxidized to dehydroascorbic acid and a triiodide complex ion is reduced to iodide ion. Reaction 1.0 illustrates the redox reaction between ascorbic acid and triiodide and Reaction 2.0 illustrates the formation of the triiodide complete. All of the spectator ions have been left out of Reaction 2.0.
Figure 1: Formula for Molarity
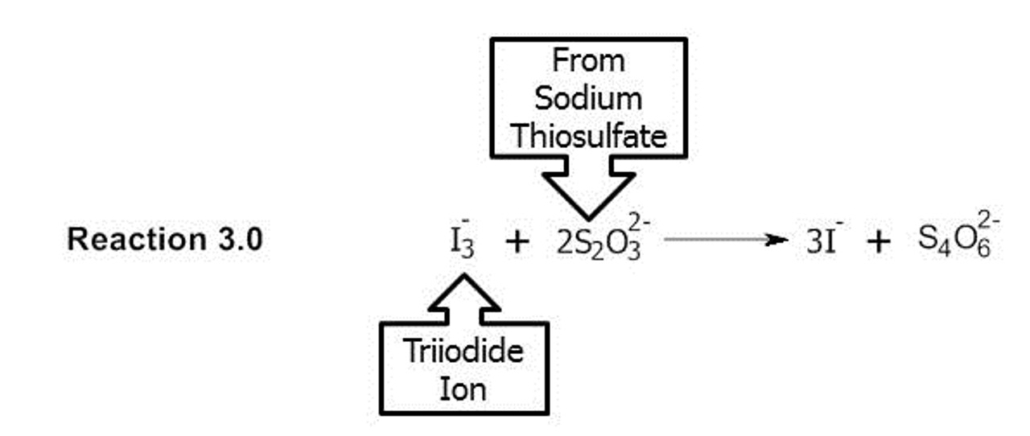
The triiodide produced during Reaction 2.0 is more than is necessary to react with all of the ascorbic acid in the juice sample (Reaction 1.0). The amount of excess triiodide will be determined by titrating with the sodium thiosulfate solution. This is known as back titration. Back titration involves creating a known amount of reagent (triiodide), reacting the known amount with the sample (juice), and the determining how much of the reagent (triiodide) is unreacted by titrating with another known solution (sodium thiosulfate) to stoichiometrically determine the amount of the analyte (ascorbic acid) is the sample (juice). The reaction that occurs between the triiodide and the sodium thiosulfate (the back titration) is illustrated in Reaction 3.0.
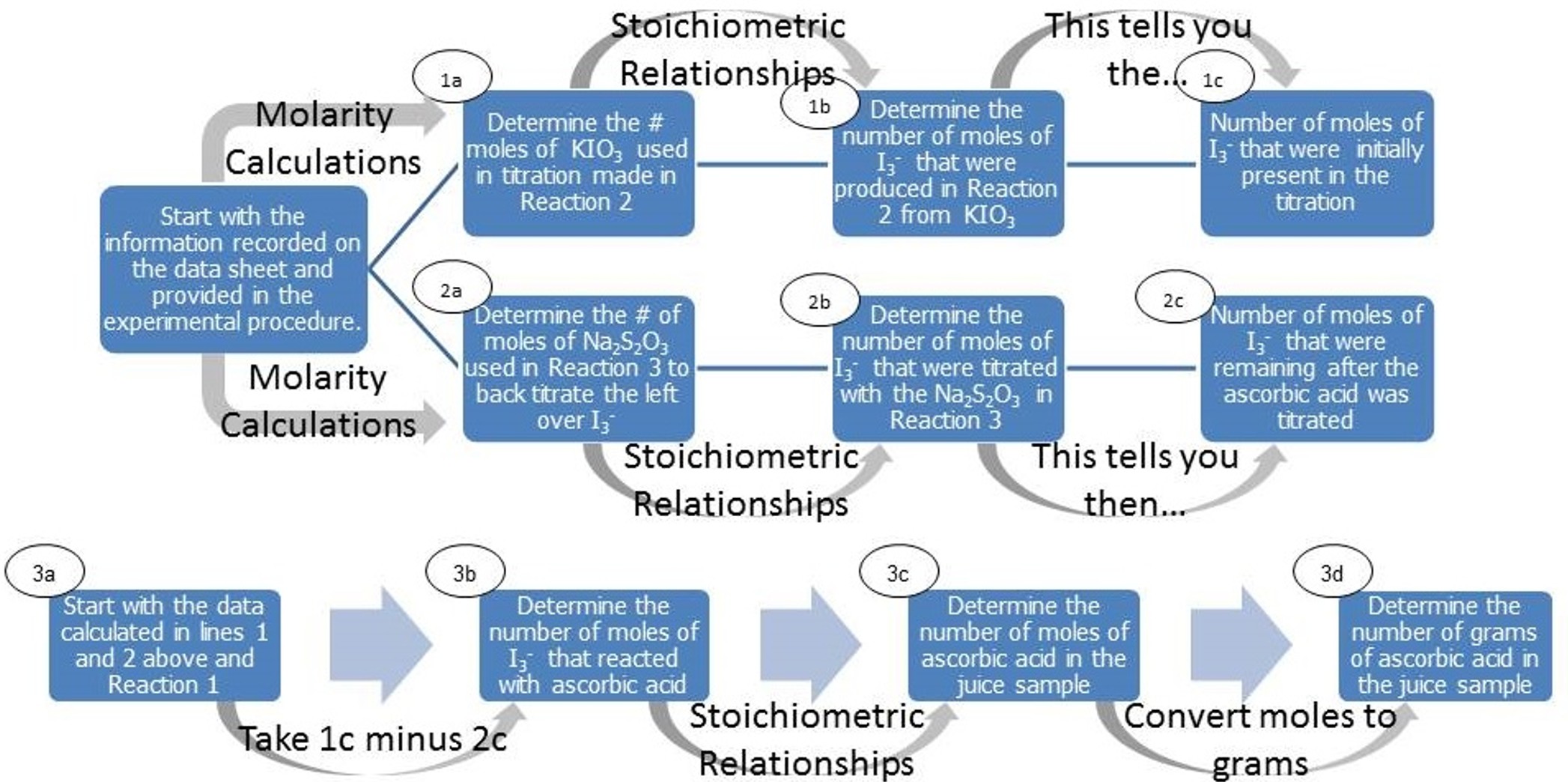
In this week’s lab, you will determine the amount of ascorbic acid, or vitamin C. A starch indicator will be used to determine the endpoint of the reaction. The starch will turn from a blue color to a light yellow to clear color when the titration is complete. First, you will prepare a sodium thiosulfate solution and calculate the concentration of the solution. Second, you will prepare a solution of potassium iodate and calculate the concentration of the solution. The concentration of the thiosulfate solution will be used to titrate the juice and determine the amount of ascorbic acid (Vitamin C) in the juice. The potassium iodate solution will be used in excess. Using the data you collected experimentally, determine how much Vitamin C is in a serving of the juice tested. Figure 2 is a flow chart to help you reason through these calculations.
A Road Map to Help With the Calculations…
Figure 2: Flow Chart for Titration Calculations (HINT: Start with the data you collected and use it to complete the set of calculations in the first line (1a-1c) and second line (2a-2c) and then complete line 3.)
Please do not be intimidated about completing these calculations. You have done every one of these calculations already in this course, just not in this sequence. Please refer to your lecture notes and textbook about gram to mole conversions, stoichiometric relationships (i.e. converting from moles of one compound to moles of another compound), molarity calculations, and unit conversions for help in completing each of these steps.
Note: It is very important that you come to lab prepared to start the experiment and that you work efficiently during lab time. However, do not rush through this lab. Take your time and be sure to record all data correctly so you have accurate and precise results.
Safety Information
The safety data sheets for each chemical we will use in this week’s lab are linked below. I encourage you to review these before coming to lab.
- Potassium Iodide is a skin and eye irritant.
- Sodium thiosulfate is not a hazardous compound.
- Potassium iodate is an oxidizer that may intensify fire. Keep away from open flames. It is harmful if swallowed and can cause serious eye irritation. This is also a teratogen that may damage fertility or fetuses. Use nitrile gloves while handling this chemical.
- Sulfuric acid is a corrosive acid that can cause damage to eyes and skin.
- Starch is a combustible dust with no classified physical or health risks under the Globally Harmonized System.
Nitrile gloves and chemical aprons are available for student use in the chemistry lab. Wear your goggles throughout this lab. Wash your hands with soap and water before leaving the lab.
Prelab Lecture Video
Click on the image to the right to watch the pre-lab lecture video for this experiment. The information in this video will help you come to the lab prepared to complete this experiment.
I created data tables for you to collect and organize your data. You can print and glue these into your lab notebook, or you can recreate them in your lab notebook using pen.
Video 1.0 – Pre-lab lecture video. Click on the image above to view the video. It will open in a new window.
Pre Lab Assignment
Answer the questions below before the start of the lab. They are due to the assignment folder on your teacher’s desk before the beginning of the lab. Failure to complete these questions before the start of the lab will result in a zero for this experiment. (12 points)
- In your own words, define the term back-titration. (1 point)
- Identify the indicator used in this week’s experiment. What color will the solution turn when the endpoint is reached? (2 points)
- What is the common term for ascorbic acid? (1 point)
- How many grams of potassium iodate are needed to prepare 250mL of a 0.02M solution? Roud your answer to 3 decimal places. NOTE: These calculations are part of Step 1 in Part B for this experiment. Include these calculations in your lab notebook. Submit them to your instructor by making a copy of the page. (3 points)
- How many grams of sodium thiosulfate pentahydrate (Na2S2O35H2O) are needed to prepare a 0.08M solution and a 200mL volumetric flask? Roud your answer to 3 decimal places. NOTE: These calculations are part of Step 1 in Part A for this experiment. Include these calculations in your lab notebook. Submit them to your instructor by making a copy of the page. (3 points)
- Describe how you would prepare the solution described in Questions #4 and 5 using a volumetric flask. Number the steps in the process for making this solution. (2 points)
Experimental Procedure
Experimental Materials
1% Starch Indicator Solution
Sodium thiosulfate pentahydrate
Potassium Iodate
0.5M Sulfuric Acid
Potassium Iodide
Juice for testing
Two 50mL Burettes
Three 250 mL Erlenmeyers
Funnel
2 100mL beakers
600mL Beaker
Burette Clamp
Burette Stand
Spatula
Scoopula
Watch Glass
100mL Graduated Cylinder
200mL and 250mL Volumetric Flasks
Hot plate with stir option
stir bar
Label Tape and Sharpe
PART A: Preparation of the Sodium Thiosulfate Pentahydrate Solution
- Prepare a 0.08M solution of sodium thiosulfate pentahydrate (Na2S2O3 x 5H2O) using a 200mL volumetric flask. You completed the calculations necessary for this step in the prelab assignment. Record the exact mass of sodium thiosulfate pentahydrate used (to 3 decimal places) on your data table.
How to Prepare a Solution Using a Volumetric Flask
- Add the solute to the volumetric flask.
- Add DI water to the flask until it is about half full.
- Swirl the flask to dissolve all of the solute.
- Add additional DI water to the line on the volumetric flask.
- Calculate the molarity of this solution and record this information on your data sheet.
NOTE: Be sure to us the exact mass of sodium thiosulfate you measured in all of your calculations. Don’t forget to also include the 5 waters in the compound in the molar mass.
- Label the flask and continue on to PART B.
Part B: Preparation of the Potassium Iodate Solution
- Prepare 250mL a 0.02M solution of potassium iodate using a volumetric flask. You completed the calculations necessary for this step in the prelab assignment. Record the exact mass of potassium iodate used (to 3 decimal places) on your data table.
- Record the mass of the potassium iodate used on the data table.
- Calculate the concentration of potassium iodate solution you prepared. Record this on your data table.
- Label the flask and continue on to PART C.
PART C: Preparing the Burettes
- Label the 600mL beaker “Waste.”
- Wash the burettes with deionized water.
- Rinse the burette with 4-8mL of the sodium thiosulfate solution prepared in PART A and drain it through the stopcock into the “waste” beaker. Label the burette “sodium thiosulfate” and place the burette in the clamp.
NOTE: Do not pour the sodium thiosulfate solution directly from the stock bottle into the burettes. Transfer the approximate amount you need first to a beaker and then add it to the burette using a funnel.
NOTE: Be sure to place any of the solutions used to “wash” the burettes in the appropriate waste container.
- Add approximately 50mL of the sodium thiosulfate solution to the burette. Allow a few drops of the solution to pass through the tip of the burette to ensure it is filled. Make sure there are no air bubbles in the burette or the tip.
- Wait a couple of minutes and record the position of the meniscus for the burette on the data sheet provided in the column for Trial 1.
NOTE: Be careful when reading the burette. Make sure you are reading the numbers and recording them properly.
-
- Repeat steps 2-5 with the second burette using the potassium iodate solution instead of the sodium thiosulfate solution.
PART D: Titration of the Juice Sample
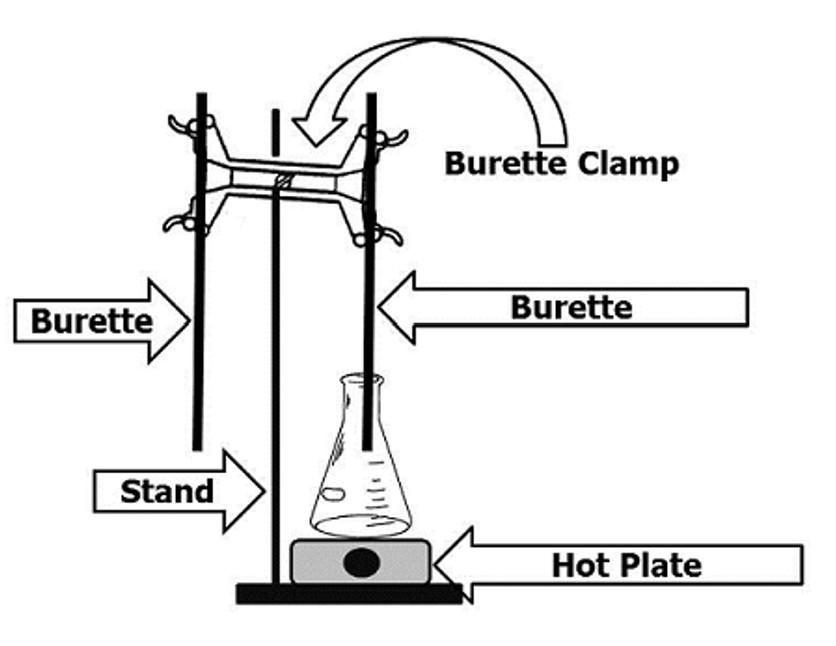
Setup the laboratory equipment as illustrated in Figure 3. One burette should contain the sodium thiosulfate solution, the other the potassium iodate solution. Label both burettes with their contents. The hot plate should be placed under the burette containing the sodium thiosulfate solution.
- Fill both burettes with the appropriate solutions and record the initial volumes for each burette on the data table.
- Measure 20mL of juice into a clean 250mL Erlenmeyer. Add 20mL of 0.5M sulfuric acid to the Erlenmeyer with the flask and swirl.
- Add 1g KI and 25mL potassium iodate solution to the Erlenmeyer containing the juice and sulfuric acid. It is ok if you add more than 25mL, just record exactly how much you add to the Erlenmeyer on the data sheet.
- Add the stir bar to the Erlenmeyer and place it on the hot plate under the burette containing the sodium thiosulfate solution. Turn on the stir option on the hot plate. DO NOT TURN ON THE HEAT.
- Begin titrating with the sodium thiosulfate solution immediately. Once the color changes to a dark yellow, add starch until the solution turns a dark blue.
- Continue titration with the sodium thiosulfate solution until the blue color fades to a light yellow.
- Repeat steps 1-4, refilling the burettes with the appropriate solutions. Record all data on the data sheet under Trials 2 and 3.
- Check the nutrition label on the juice. Record the amount of juice in one serving and the amount of Vitamin C in one serving of juice on your data table. The daily recommended amount of Vitamin C for adults over the age of 19 is 75mg for women and 90mg for men.
Figure 3: Titration Setup
Waste Disposal
- Reduce any unused potassium iodate from the burette by adding excess KI to the potassium iodate solution (at least 1g for every 20mL of potassium iodate solution). Add a few drops of sulfuric acid to the mixture. Titrate the potassium iodate with sodium thiosulfate until the yellow-brown color has disappeared. Flush this solution and any solutions that were titrated during Trials 1, 2, and 3 for this experiment down the drain with lots of water. Allow the water to run for approximately 5 minutes after disposing of the titrated solutions.
- Place all of the 250mL Erlenmeyers obtained from the cabinet in the bucket on your instructors desk.
- Wash the burette with soap and water (conducting the final rinse using distilled water) and return it to where it was originally obtained.
- Wash any of the glassware used from your drawer with soap and water (conducting the final rinse using distilled water) and return it to your drawer.
- Place your hot plate in the storage cabinet from where it was obtained.
- Wash your stir bar with soap and water (conducting the final rinse using distilled water) and return it to your instructor.
Post Lab Assignment
Items To Include In Your Lab Notebook:
Once you have completed this experiment and recorded all of your data in your laboratory notebook:
- Write the discussion and conclusions sections for the lab. The questions in the post-lab assignment will help you process the data from this lab. Be sure to include all of the calculations from the post-lab assignment in your lab manual.
- Include all calculations for determining the concentration of these three solutions. Ensure everything is neat and easy to follow. Please circle and label the final calculated concentration for each solution.
- Determine the average amount (in mg) of Vitamin C in your juice sample.
- Calculate the percent difference between your experimental findings and the juice sample. Comment on the difference between your experimental value and the amount of Vitamin C reported by the manufacturer. You can find the manufacturer information in the nutrition label on the side of the bottle.
- Enter your average amount per serving (in mg) of Vitamin C in the classroom data table. This is provided on Brightspace under Content -> Materials -> Laboratory Materials -> Look for the link to “Redox Titration of Vitamin C Class Experimental Data Table.”
- Complete the post-lab assignment and submit it to your instructor.
Are you not sure how to start this work? Please review the prelab lecture video and the Calculations Road Map information provided in the introduction for more information. I provide guidance on how to complete this work in these materials.
Redox Titration of Ascorbic Acid Post-Lab Assignment (15 points)
Using the information you gathered during today’s lab, answer the following questions. This assignment is due at the start of the next lab period.
- Does the amount of Vitamin C you calculated experimentally per serving match the amount in each serving as stated on the nutrition label on the juice bottle? Mathematically support your conclusion. Show calculations for 1 trial. Also provide the final calculations for Trials 2 and 3 and the average for all 3 trials. You can include a copy or photo of your calculations for Trial 1 from your lab notebook for this question. (6 points)
- What is the percent difference between your experimental results and the amount of Vitamin C reported on the label of the juice? What does this information tell you? (3 points)
- Look at the classroom data. How does the average of the class’s data compare to the reported value (i.e. calculate the percent difference). Comment on the accuracy and precision of this data. Include the mean, standard deviation, and range for this data in your analysis. Show all of your work. (4 points)
References
Global Safety Management, Inc. “potassium iodide.” Datasheet, Dec. 2014. https://www.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-p/S25864.pdf
Global Safety Management, Inc. “starch.” Datasheet, Jan 2015. https://www.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-s/S25582.pdf
National Institutes of Health. (2021, Mar 6). Vitamin C Fact Sheet for Health Professionals. National Institutes of Health. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/
N.D. (2013). Determination of Vitamin C Concentration by Titration. University of Canterbury, College of Science. https://www.canterbury.ac.nz/content/dam/uoc-main-site/documents/pdfs/d-other/Determination-of-Vitamin-C-Concentration-by-Titration.pdf
Sigma Aldrich. “potassium iodate.” Datasheet, March 2025. https://www.sigmaaldrich.com/BE/en/sds/aldrich/438464?srsltid=AfmBOoqzdo_BrgWbrC7LNJAXZ-g4stHVvFakHstuHke-sTobBFPADVHz
Sigma Aldrich. “sodium thiosulfate.” Datasheet, Sept 2024. https://www.sigmaaldrich.com/US/en/sds/sigald/217263
Sigma Aldrich. “sulfuric acid.” Datasheet, Sept 2024. https://www.sigmaaldrich.com/US/en/sds/aldrich/339741?srsltid=AfmBOorOhzcu8A5Q2zijEWXqm88AyqZhOZi99cvp2WMeS5n_Gey9s6Cb
This page was published on March 25, 2025 and last updated on March 27, 2025.
©Catherine Haslag 2025. All Rights Reserved.