What’s in my blood?
Objectives
- Express numbers in scientific and normal notations
- Write the name and formula for a compound.
- Determine the molarity of a solution.
- Convert between grams and moles for a substance.
- Calculate concentration using percent by mass and by volume.
- Conduct parts per million concentration calculations.
- Express measurements in and convert between metric units.
- Explain the components and purpose of blood in the body.
Introduction
Blood is a complex homogeneous mixture. It contains ions, dissolved gases, cells, biological chemials, and bodily waste. It is the major biological highway in the body. It transports oxygen (O2) from the lungs to organs and other tissues in the body. It collects carbon dioxide (CO2) and brings it to the lungs. We expel the carbon dioxide when we exhale. Blood transports nutrients from the intestines to the liver and other organs. It collects wastes and transports them to the kidneys for excretion as urine. Our blood also carries hormones, antibody proteins, and other things the body needs to survive.
The human body contains on average 5 to 6 liters of blood Approximately 45% of our blood volume is made of cells. These types of cells are described below:
- Erythrocytes – red blood cells that are responsible for transporting dissolved oxygen and carbon dioxide.
- Leukocytes – white blood cells that are part of the immune system and responsible for immune function.
- Blood platelets –responsible for clotting blood.
The other 55% of blood is made of plasma, which contains mostly water, ions, nutrients, and wastes.
Watch the video below to learn more about our blood, what it is made of, and what it does for us.
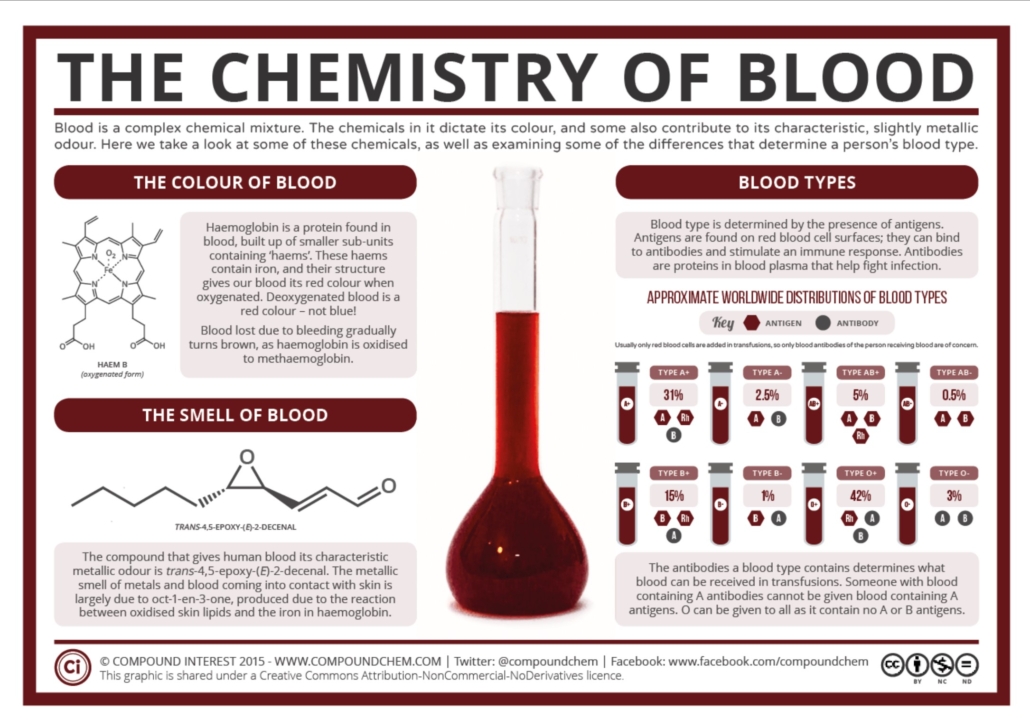
The image below, obtained from CompoundChem, provides more information about the chemical make-up of blood and how blood typing works.
Assignment
Answer the following questions using the information provided in the assignment introduction, your textbook, and the concepts we have learned in this class. Your assignment must be handwritten. Typed submissions will not be accepted and will earn a zero. You must show all of your work on the calculations to earn credit. Upload your completed assignment to the “What’s in my blood?” assignment folder on Brightspace. This assignment is worth 25 points.
- What are 5 chemicals found in your blood? (1 point)
- What are 4 functions blood serves in the body? (2 points)
- A patient has a blood volume of 5.2L. Blood contains many ions and chemicals, including sodium and potassium. A normal sample of blood has a sodium concentration of 0.139M and a potassium concentration of 0.0048M. Using this information, answer the following questions:
- How many total grams of sodium are present in the patient? (2 points)
- How many total grams of potassium are present in the patient? (2 points)
- Normal saline solution is used in intravenous drips for patients to keep them hydrated. This solution is created by dissolving 9.0g of sodium chloride in water to make 1000mL of solution. Using this information, answer the following questions:
- What is the sodium concentration (in M) for normal saline solution? (2 points)
- How does the concentration of sodium in saline solution compare to the concentration of sodium in the human body? (1 point)
- One common intravenous therapy involves the administration of Hartmann’s solution to replace lost body fluid and mineral salts. Hartmann’s solution is a solution with several salts, including potassium chloride, calcium chloride, sodium chloride, and sodium lactate (NaC3H5O3). Determine the mass of each of the following solutes used to make 750mL of Hartmann’s solution: (2 points each, 8 points total)
0.0050M potassium chloride
0.0020M calcium chloride
0.102M sodium chloride
0.0260M sodium lactate
- The average adult contains 5.1×106 erythrocytes (red blood cells) per liter. Write this number in normal notation. (1 point)
- The average diameter of an erythrocyte is 0.0077mm. How many kilometers long is a erythrocyte? Write your answer in scientific notation. (2 points)
- The average density of whole blood is 1.06g/mL. Using this information and the information/calculations you completed in problem #3, answer the following questions:
- What is the concentration of sodium in the blood? Express your answer in parts per million. (2 points)
- What is the concentration of potassium in the blood? Express your answer in parts per million. (2 points)
References
Compound Interest (2015). Halloween Special: The Chemistry of Blood. Compound Interest. https://www.compoundchem.com/2015/10/29/blood/
Crash Course. (2015, August 3). Blood, Part 1 _ True Blood: Crash Course Anatomy and Physiology #29 [Video]. YouTube. https://www.youtube.com/watch?v=HQWlcSp9Sls
Dillon, S.R. (n.d.) The Analysis of Human Blood. Chemistry for Liberal Studies – Forensic Academy. https://www.chem.fsu.edu/chemlab/chm1020c/Lecture%2012/01.php
Khan Academy. (n.d.) Components of Blood. Khan Academy. https://www.khanacademy.org/science/biology/human-biology/circulatory-pulmonary/a/components-of-the-blood
The questions included at the end of this assignment were modified from:
Gries, T. (2012). What is in my Blood? ChemConnections Activity Workbook. (pp 39-42). W. W. Norton & Company.
This page was published on January 29, 2023, and last updated on August 8, 2023.
©2023 Catherine Haslag. All Rights Reserved.