Osmosis and Diffusion
Objectives
- Define a hypotonic, hypertonic, and isotonic solution.
- Explain how osmosis.
- Explain the difference between osmosis and diffusion.
- Explain the role of osmotic pressure in osmosis.
- Draw conclusions based on experimental data.
Related Textbook
Please read section 16.10 of your textbook before beginning this activity. The textbook provides terms, concepts, and other important background information to help you succeed on this assignment.
Introduction
This week’s laboratory activity is a case study on diffusion and osmosis. Be sure to read through the background information provided here before beginning the case study. The list of experimental materials listed for this laboratory are used in the experiment explained in part 2 of the case study.
NOTE: This experiment takes at least 14 hours to complete (12 hours of it are waiting for the experiment to work). DO NOT wait until the last minute to complete this experiment. Please plan accordingly.
Solutions are made of two components: solutes and solvents. The amount of solute that will dissolve in a specific amount of solvent will determine the solution’s concentration. There are many ways to measure concentration, including molarity, parts per million, and in percent concentration. When there is a difference in concentration for a solution, solute, and solvent molecules will migrate to even out the concentration. This difference in concentration of solution(s) is called a gradient.
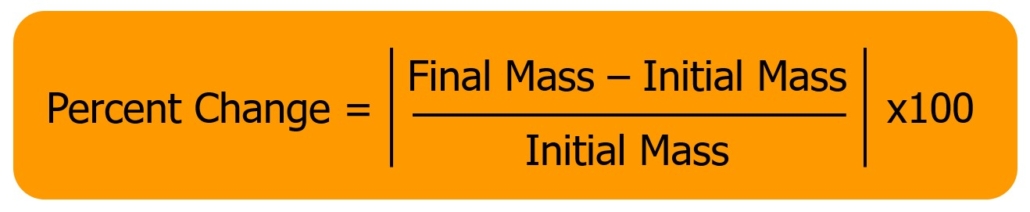
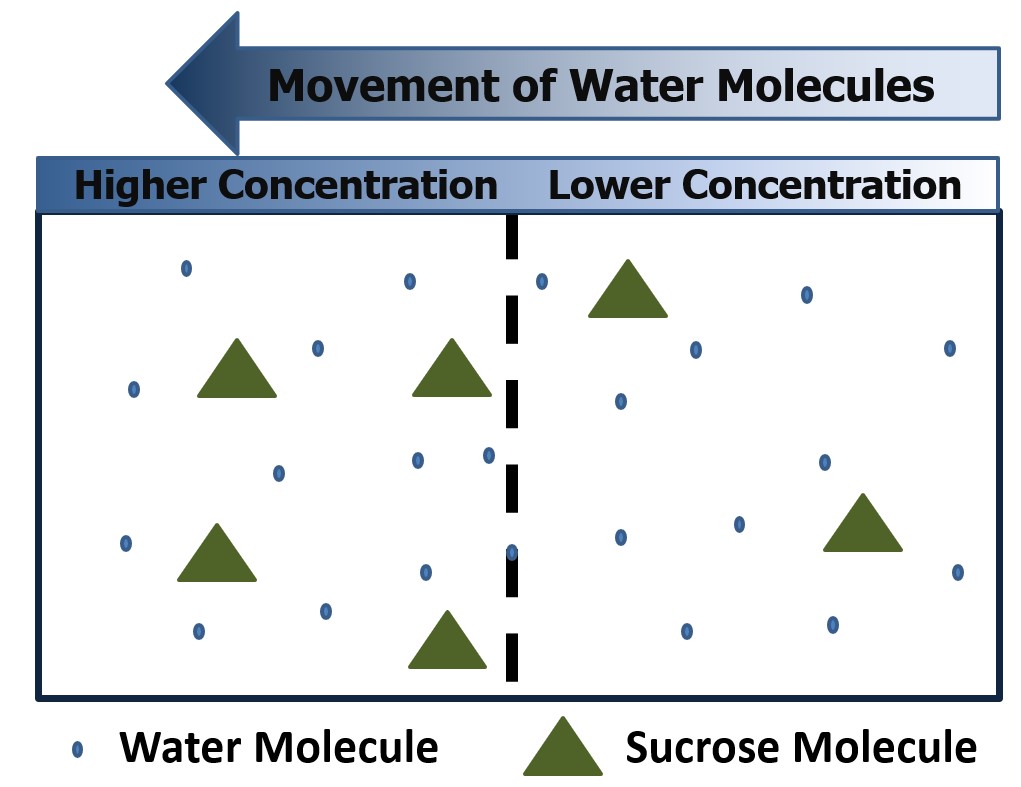
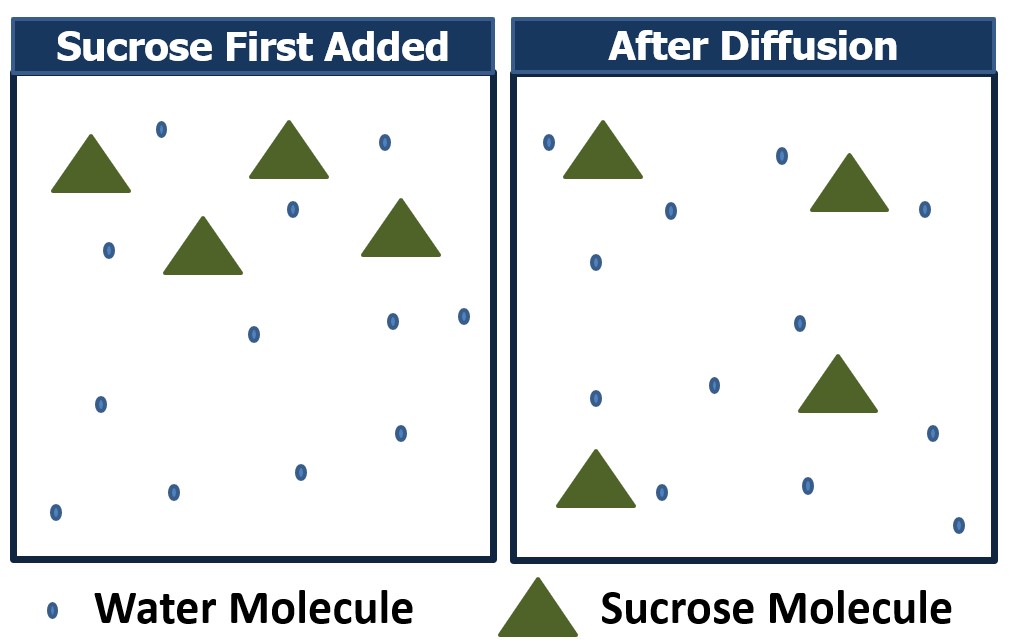
There are two ways this movement can occur. Diffusion involves the movement of molecules from a region of high concentration to an area of low concentration. This can occur between a solute and solvent, as illustrated in Figure 1, or between two gasses. This process does not involve the presence of a semi-permeable membrane and tends to simply spread the molecules out until the concentration is even throughout. Figure 1 illustrates how diffusion occurs. On the left, sucrose has just been added to a water solution and is concentrated in one area. Over time, these molecules diffuse (spread out) throughout the entire container, resulting in a more even concentration of sucrose in the solution. The diffusion rate can be increased by shaking or mixing the solution to increase the velocity of the molecules being combined. When water molecules move across a semi-permeable membrane due to a difference in concentration, the process is called osmosis. Osmosis is a type of diffusion; however, it specifically involves water movement across a semipermeable membrane. A semipermeable membrane is designed to allow the movement of some molecules through it while blocking the movement of others. An example of this is shown in Figure 2. As illustrated, the water molecules can move freely through the semipermeable membrane while the sucrose molecules are unable to cross because pores in the membrane are not big enough to accommodate the sucrose molecules.
In the case of Figure 2, the left side has a higher concentration of sucrose than the right side, so water (the solvent) will flow from the right side across the membrane to the left side until the concentration of sucrose in solution is the same on both sides of the semipermeable membranes. Water molecules move back and forth across the membrane; however, the rate of water movement will be greater in the direction of the higher concentration of solute, causing the fluid level to increase on the side with the most solute.
Osmotic Pressure drives this movement of solvent from one side of the semipermeable membrane to the other. The solvent molecules will move from one side of the membrane to the other until the osmotic pressure on both sides is equal. The greater the number of dissolved particles (i.e. the greater the concentration), the greater the osmotic pressure will be.
In Figure 2, the solution on the left side of the membrane has a higher concentration of solute than the solution on the right side, so the solution on the left side is hypertonic. Since the right side has a lower solute concentration, the solution is hypotonic. The solutions are isotonic when the same amount of solute is present in solution on both sides of the semipermeable membrane.
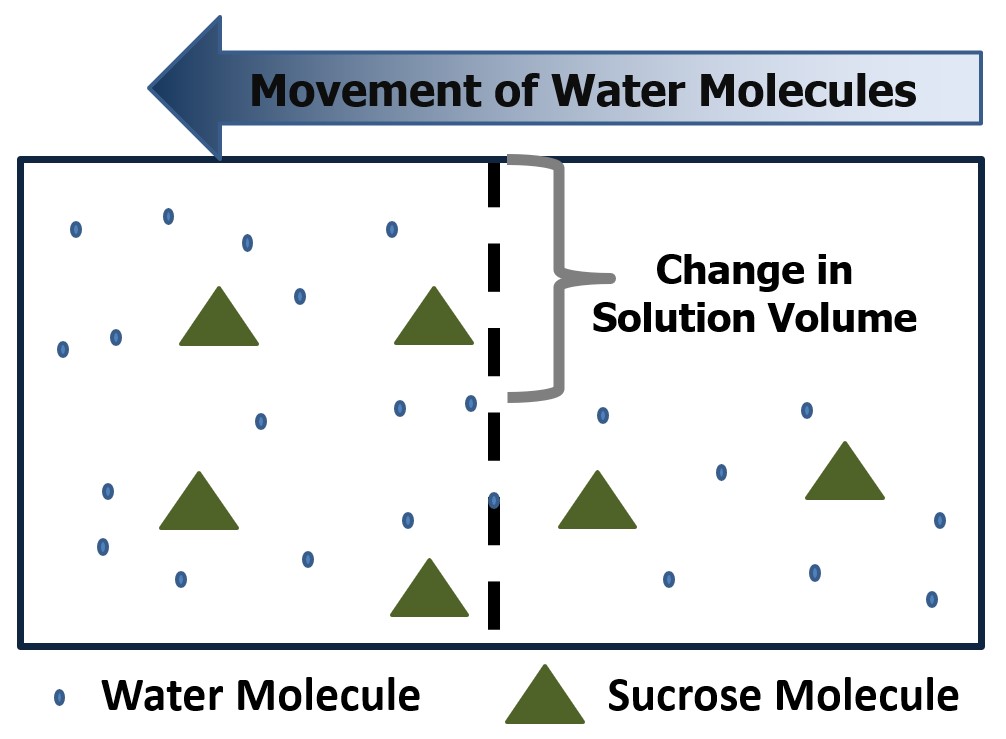
When the water flows from one side of the semipermeable membrane to the other, a difference in solution volume will occur. The solution volume will decrease on the side of the membrane that contains fewer solute molecules (the hypotonic side) and increase on the side that contains more solute molecules (the hypertonic side) Figure 3 illustrates this idea. This solvent movement causes the solute concentration to decrease until the osmotic pressure reaches the point where water molecule movement will still occur back and forth across the membrane, but the total solution volume will no longer change.
An example of a semipermeable membrane in animals and humans is the cell membrane, which allows for some substances to pass into and out of the cell while preventing the passage of others. Wastes and nutrients can cross the cell membrane while other molecules are denied such passage. In plants, osmosis is partially responsible for water absorption from the soil and the movement of the liquid from the roots to the plant’s leaves.
In this week’s lab, you will read a case study and complete an experiment demonstrating how osmosis works and how it is affected by changing a concentration gradient. You will record the gummy bears’ initial and final mass and calculate the mass change for each gummy bear using the percent change formula (Figure 4.0).
Figure 4.0 – Percent Change Formula
You will organize your data for this lab into a data table. Click below for a copy of the data table you will need for this lab. It will download to your computer. If you have trouble downloading it, check to ensure blockers are disabled. ALL values in this lab MUST be measured in metric units.
Safety Concerns
There are no safety concerns for this experiment.
The gummy bears used in this experiment should not be consumed.
Figure 1 – How diffusion works.
Figure 2 – How osmosis works.
Figure 3 – A representation of solvent movement due to osmotic pressure.
Experimental Materials
Chemicals and Supplies
3 gummy bears
3 glasses of the same size
Sodium Chloride (table salt)
Plastic Wrap or Aluminum Foil
Spoon
4 Rubber Bands
Paper Towels
Distilled Water
Masking Tape
Sharpe Marker
Digital Balance
Watch
Case Study1
NOTE: The case study and assignment questions were copied directly from Bonney, 2014 with minor revisions.
Part I – Introduction to Diffusion and Osmosis
Sue: “Hey Jude, what did we learn in Mr. Phillotson’s biology class today? I missed it because I wasn’t feeling well.”
Jude: “Today we mostly discussed diffusion and osmosis.”
Sue: “What are diffusion and osmosis?”
Jude: “Well, diffusion is the movement of molecules of a substance from an area of high concentration to an area of lower concentration. When molecules do that it’s called moving down a concentration gradient. Mr. Phillotson demonstrated diffusion in class by placing a few drops of green food coloring in a glass of water. At first most of the water was clear with a small amount of dark green food coloring concentrated in the center where he had placed the drops, but over a few minutes the molecules of food coloring spread out in the water until they were evenly distributed among the water molecules and the entire glass appeared a light green color. He said the same thing happens in the air when someone sprays perfume inside a room. At first the molecules of perfume are concentrated and strong-smelling in a small area, but over time they diffuse through the air in the room until they are evenly distributed and the entire room smells weakly of perfume.”
Sue: “Where does the energy come from to move the molecules during diffusion? Did Mr. Phillotson mention that?”
Jude: “Yes, actually, he did. He said that molecules move down their concentration gradient spontaneously, without any work being done, so no energy input is required. Diffusion of molecules can even occur through a membrane, as long as the membrane has holes or pores that will allow those molecules to pass through. Mr. Phillotson said that if molecules diffuse down their concentration gradient through a biological membrane, such as the plasma membrane of a cell, that is called passive transport. Passive transport does not require energy input from the cell.
Sue: “So, passive transport moves molecules across a cell membrane and does not require energy input from the cell because the necessary force is provided by the concentration gradient?”
Jude: “Correct. Molecules can also be moved across a cell membrane against their concentration gradient, but that would require energy input. Mr. Phillotson said that process is called active transport, and that we would learn more about it later. ”
Sue: “Ok. I think I understand diffusion now. So, what is osmosis?”
Jude: “Osmosis is the diffusion of water across a selectively permeable membrane. During osmosis water moves from an area of higher concentration of water molecules to an area of lower concentration of water molecules.
Sue: “If water molecules move down their concentration gradient during osmosis, that must happen spontaneously and not require energy input, right?”
Jude: “Correct.”
Sue: “I’m confused, though. I thought all water was the same. How can there be different concentrations of water?
Jude: “Mr. Phillotson explained that often, whether inside a living organism or in a laboratory experiment, water contains other molecules, or solutes. The higher the concentration of solutes, the lower the concentration of water. Do you understand, Sue?”
Sue: “I think so… but… did Mr. Phillotson happen to do a demonstration of osmosis?”
Jude: “Actually, he gave us a homework assignment to help us understand osmosis, and said we will go over another example in class next time, after we discuss the homework.”
Part II – Osmosis and Gummy Bears
You will need to take photos of your gummy bears before and after the experiment begins and a photo of them in their labeled glasses, with the proper contents added to each glass at the start of the experiment. A piece of paper that includes your name, CHEM 1000, and the semester you are taking this class (for example, if you take this during the fall semester in 2023, write “Fall 2023” on the paper) should also appear in these photos. This information must be handwritten, legible, and large enough to be clearly read on the piece of paper. The assignment tied to this laboratory will not be graded unless all of these photos have been submitted to the assignment folder on Brightspace.
Sue: “Homework?! Ugh. What is the assignment?”
Jude: “Each of us was given three gummy bears and these instructions:
Step 1. Measure and record the starting mass of all three gummy bears. Record this data using the data table.
Step 2. Gather three glasses of the same size, and label them #1, #2, and #3.
Step 3. In glass #1, add one inch of water at room temperature and place one of the gummy bears inside.
Step 4. In glass #2, add 1/4 inch of salt and ¾ inch of water at room temperature, as well as one gummy bear.
Step 5. In glass #3 add nothing except the third gummy bear.
Step 6. Cover all three glasses with plastic and leave for at least 12 hours.
Step 7. After 12 hours, measure the final mass and record it on the data table.
Step 8. Determine each gummy bear’s mass change and record your results on the data table.
Part III – Osmosis in Animal Cells
The experiment that Mr. Phillotson’s class conducted simulates what happens when living cells, such as your own red blood cells (RBCs), are placed in solutions of different tonicities. The gelatin in gummy bears forms a structural matrix that acts somewhat like a selectively permeable membrane, allowing passage of certain molecules but not others. Water will move in or out of the gummy bear matrix when placed in solutions of different tonicities in order to obtain equilibrium. The plasma membrane that surrounds RBCs and other animal cells is similarly selectively permeable; water will move across the membrane more readily than will solutes such as sodium chloride (NaCl).
Like a gummy bear, an animal cell’s plasma membrane will shrivel if there is a net water loss from inside the cell. However, unlike a gummy bear, if there is a large net influx of water into the cell, the plasma membrane may break, and the cell will be destroyed. The bursting of the cell in that situation is called lysis.
Part IV – Osmosis in Plant Cells
Unlike gummy bears and animal cells, the cells of plants are surrounded by rigid cell walls. These cell walls will prevent cells from bursting if there is a large net water movement into the cell. However, when a plant cell swells due to a net influx of water, the cell wall can only expand so far before exerting pressure back on the cell, which is called turgor pressure. Many plants depend on turgor pressure and the firm, or turgid, state it creates to provide structural support. If you have ever seen a houseplant or a stalk of celery go limp after not receiving water for a while, you have observed plant cells that have lost turgor pressure and become flaccid. Conversely, a significant net water loss from inside a plant cell can cause its plasma membrane to pull away from the inside of the cell wall; this state is called plasmolysis.
Waste Disposal
- Place the gummy bears in the trash.
- Flush the sink with warm water after disposing of the chemicals.
- Wash any other glassware used with soap and water once the experiment is complete.
Assignment
Once you have read the case study provided on the following pages and completed the experiment explained in Part II of the case study:
- Submit the completed data table for this experiment.
- Submit the 3 photographs of the gummy bears as outlined at the start of Part 2 of the case study.
- Complete all the questions below about the case study and your experimental results. Please number your answers to each question so I can clearly and easily follow your work. Your answers can be handwritten or typed. This assignment is worth 10 points.
Part 1 Questions
- Define the terms diffusion, passive transport, active transport, and osmosis. In each of your definitions, describe the role of a concentration gradient. (2 points)
- Biological membranes are said to be selectively permeable (or semi-permeable). What does this term mean, and how does this affect the way that molecules can move through cellular membranes? (0.5 Point)
- Which molecule type is more likely to quickly pass through a cellular membrane via simple diffusion, polar or nonpolar? Why? (You may need to use information from your textbook or other course materials to answer this question.) (1 point)
Part 2 Questions
- Define the following terms: hypertonic solution, hypotonic solution, and isotonic solution. (1.5 points)
- Which of the terms from the previous question describes the solutions in each of the glasses the gummy bears placed into? (1 point)
Part 3 Questions
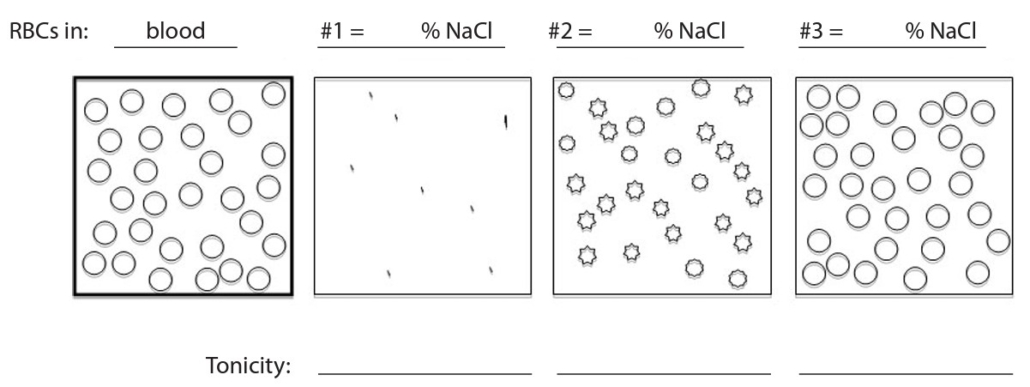
The solute concentration in blood is equivalent to 0.9% NaCl. In the lab section of Mr. Phillotson’s class, students viewed a blood sample with a microscope. Next, several drops of blood were added to three different solutions: 0.09% NaCl, 0.9% NaCl, and 9% NaCl. (Solutions can be made by adding 9.0 g of NaCl to 100 ml of water to produce 9% NaCl, then mixing 10ml of this solution with 90 ml of water to produce 0.9% NaCl, and mixing 10ml of this solution with 90ml of water to produce 0.09% NaCl).
- In the figure below, the panel on the left shows how the RBCs appeared in the blood when viewed at 400× magnification. Label the three other panels by indicating which shows RBCs added to 0.09% NaCl, to 0.9% NaCl, and to 9% NaCl. Below the panels, indicate whether each solution is isotonic, hypertonic, or hypotonic in relation to RBCs. (2 points)
2. Hospital patients often receive medications, nutrients, and water intravenously (IV), which means they are injected directly into the patient’s veins through a needle. IV fluid is not pure water, but is instead a saline solution (water containing NaCl). What do you think is the appropriate NaCl concentration for IV fluid? Why? (1 point)
3. What would happen to a cell if pure water was used as IV fluid instead of saline solution? (1 point)
References
(1) Bonney, K. M. From Gummy Bears to Celery Stalks: Diffusion and Osmosis. J.Coll. Sci. Tea. [Online] 2014, 43(6), 72–76. https://doi.org/10.2505/4/jcst14_043_06_72 (accessed August 3, 2023)
This page was created on August 2, 2023, and was last updated on February 2, 2024.
©2023 Catherine Haslag. All Rights Reserved.