Physical Properties of Matter Experiment
Objectives
- Express numbers in scientific and normal notation.
- Express measurements in and convert between metric units.
- Determine the accuracy and precision of a set of data.
- Explain how density is determined using the direct and indirect methods.
- Calculate the density of an object.
- Distinguish between a physical and chemical property.
Related Textbook
Please read chapter 2 and sections 3.5 and 3.6 of your textbook before beginning this activity. The textbook provides terms, concepts, and other important background information that will help you succeed on this assignment.
Introduction
Measurement and Unit Conversion
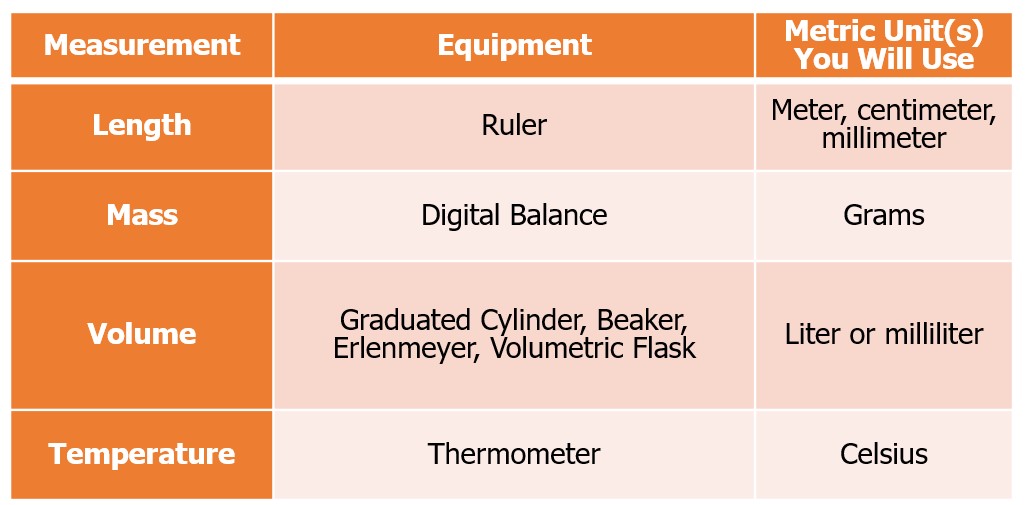
The basis for obtaining good results in the laboratory is the collection of accurate data. This includes carefully following the experimental procedure, recording observations, and documenting all relevant measurements and the correct units. Most of us are familiar with the English System of measurement, which utilizes units such as feet, gallons, acres, and pounds; however, in the laboratory, only metric units are utilized. The metric base units for the different measurement forms we will use in the laboratory are provided in Figure 1.0.
Figure 1.0 – Common laboratory equipment, what is measures, and common metric units you will use in this class.
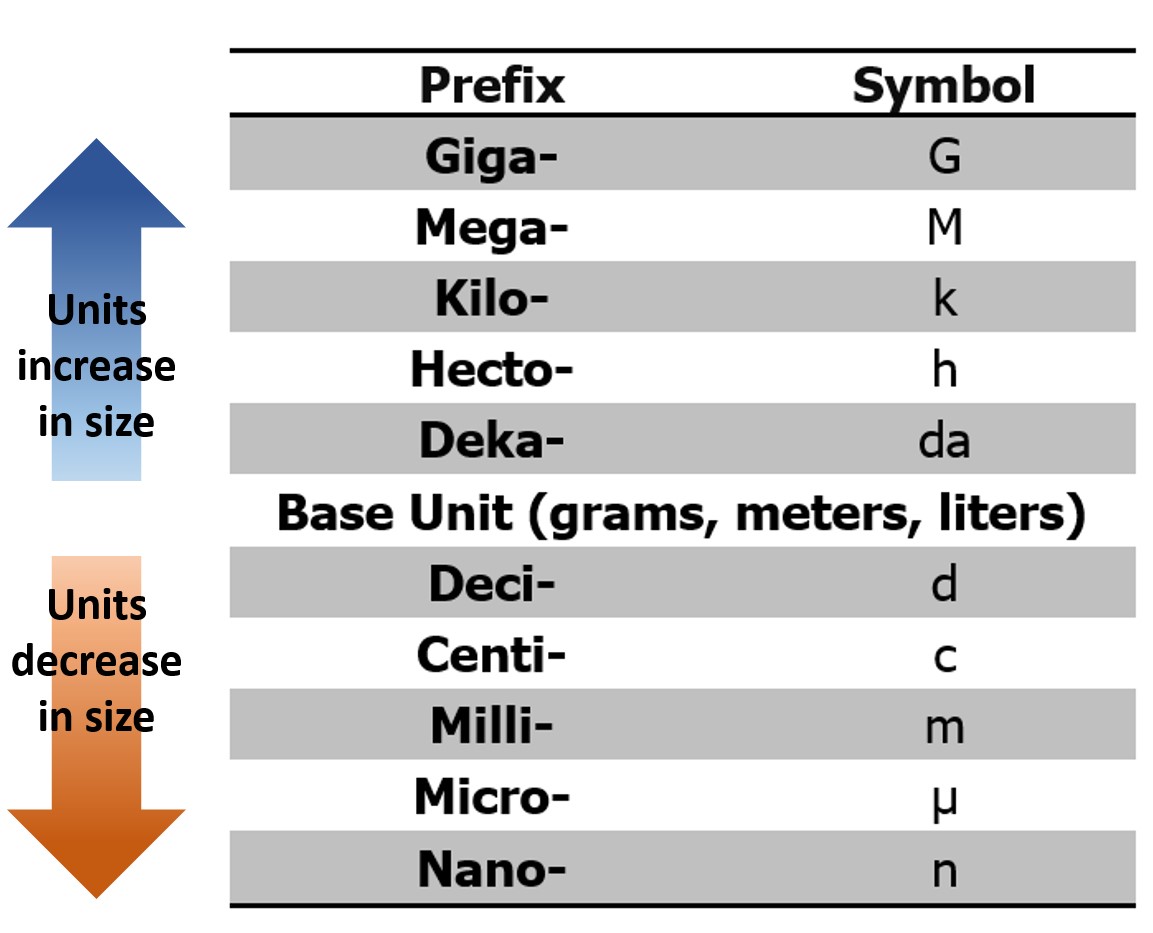
Figure 2.0 – Prefixes used with metric measurements.
Prefixes are used in combination with each of these base units. The metric system is based on a power of 10 system, making it much simpler to convert from meters to centimeters (1m = 100cm), as opposed to converting from feet to inches (1ft = 12in). It is important that you know these prefixes, record units with all data collected, and are able to convert between the different units of measurement. A list of these prefixes and their values are provided below in Figure 2.0.
It is also important to keep in mind the size of the object you are measuring with regard to the appropriate units that should be used. When measuring with the English System, feet are used to measure the height of an adult human, and miles are used to measure the distance on a road trip. In the metric system, the height of an adult human is typically measured in meters or centimeters while the distance from Austin to Minneapolis would be measured in kilometers.
Correct Measurement of Volume
Liquids will often form a slight dip at the surface when they are placed in a container. The bottom of this dip is called the meniscus. When measuring a liquid’s volume, measuring at the bottom of the meniscus is important. Place the container on the bench top and lower yourself to eye level with the meniscus to do this. Figure 3.0 illustrates this procedure.
Accuracy and Precision
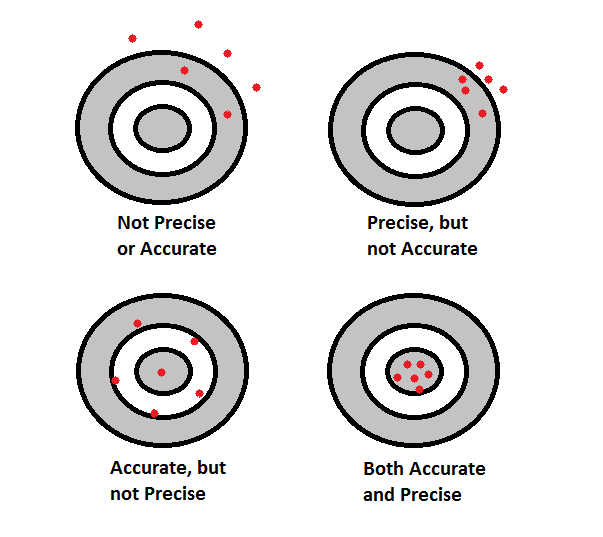
Accuracy and precision are important in determining the validity of the data you collect. Figure 4.0 discusses accuracy and precision. Accuracy refers to a data set’s closeness to the actual true value. Precision refers to the degree to which a data set shows the same results, or is reproducible. Data can be accurate and not precision, precise and not accurate, neither, or both. A set of data is valid if it is accurate and precise. Figure 5.0 illustrates this concept. In this case, the known value is the center of the bullseye. When the data points are not close to the center of the bullseye (the true value), then they are not accurate. This is shown by the two bullseyes at the top of Figure 5.0. If the data points are not close to each other, as illustrated in the top and bottom bullseyes, then the data is not precise. If the data points are close to the bullseye and clustered together, as illustrated in the bullseye at the bottom right of Figure 5.0, then the data is both precise and accurate.
Density
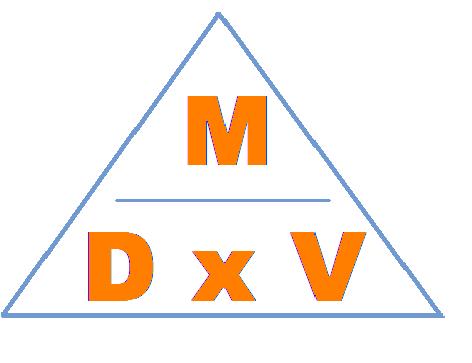
The density (D) of an object is determined by taking the mass (m) of the object and dividing it by the object’s volume (v). Density for solids and liquids is typically expressed in terms of grams per milliliter (g/mL) or grams per cubic centimeter (g/cm3). The density of a gas is expressed in g/L. Figure 6.0 represents the formula for density. A substance’s density at a specific pressure and temperature is a constant physical property no matter the size of the sample; therefore, density can be used to help identify a solid or liquid.
A liquid with a lower density will float on a liquid with a higher density. This is why ice (density = 0.92g/mL) floats on water (density = 1.0g/mL). Liquids with differing densities will form separate layers unless they are very soluble in each other. For instance, if several liquids of differing densities are placed in a beaker, the liquid with the greatest density will be located on the bottom of the beaker and the liquid with the lowest density will be on the top.
A similar phenomenon will occur with solids and liquids. A solid object will float on a liquid if the density of the object is less than the density of the liquid. A solid object in a series of layers will float within a layer that has the same density or will float between two layers with densities higher or lower than its own density.
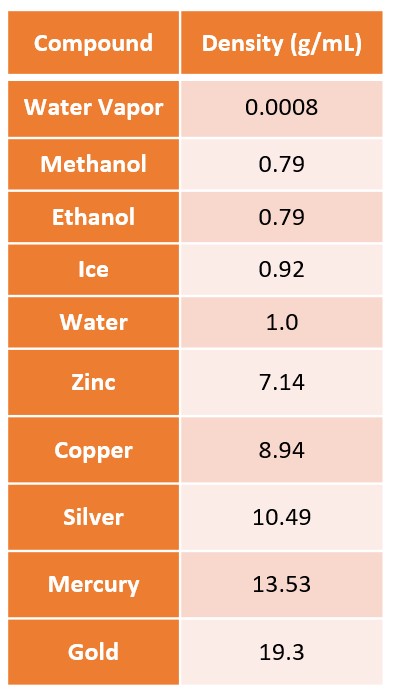
A material will have different densities in its different phases, or states of matter. Most matter is denser in the solid phase than the liquid phase and the liquid phase is denser than the gas phase. Water differs from most substances in that ice is less dense than liquid water and will float. Figure 7.0 provides the density of several different substances.
Remember: the formula for volume is length x width x height
Physical Properties and Changes of Matter
Measurements (such as volume and length), density, color, luster, taste, texture, odor, melting and boiling points, and shape are all examples of the physical properties of matter. A physical change in matter can involve changing the size, shape or phase state of the object; however, a physical change does not alter the object on a molecular level. For instance, bending a copper wire changes the shape of the wire, but it does not change the nature of the metal. It’s still a copper wire. Boiling water does not change the structure of water, but rather converts it from the liquid phase to the gas phase.
Equipment Notes: Using the Digital Balance
Be sure to read the directions that come with your digital balance, so you know how to use it properly. The digital balance should also read all zeros on the screen before any items are placed on the balance. Be sure to learn how to tare and zero out the balance before using it. The directions provided with your balance should explain these processes. When recording data, always write down ALL of the digits that appear on your digital balance, even if they are zeros. All the digits matter when using a digital device.
In this week’s experiment, you determine the density of several household liquids. You will organize your data for this lab into a data table. Click below for a copy of the data table you will need for this lab. It will download to your computer. If you have trouble downloading it, check to ensure blockers are disabled.
The Physical Properties of Matter Data Table
Experimental Procedure
Chemicals and Supplies
Digital Balance
25mL Graduated Cylinder
100mL Graduated Cylinder
Clear drinking glass
Laundry detergent
Isopropyl Alcohol
Oil (see step 1)
Milk
Above: Figure 3.0 – How to measure using the meniscus.
Below: Figure 4.0 – Accuracy and Precision Explained
Above: Figure 5.0 – A visual representation of accuracy and precision.
Below: Figure 6.0 – Density Formula
Below: Figure 7.0 – A visual representation of accuracy and precision.
Determining the Density of a Solution
- Determine the density of 20mL of each of the following compounds:
- Laundry Detergent
- Oil (Mineral oil, vegetable oil, olive oil, etc. whichever you have around your house, but specify which type of oil you are using.) DO NOT USE any kind of machine oil (motor oil, etc.)
- Isopropyl Alcohol
- Milk (specify the type of milk, i.e. whole, 2%, skim, etc.)
Based on the information discussed in lecture, develop your own experimental procedure to determine the density of each of these liquids. Record your experimental procedure on the data table.
- Once the density of each solution has been determined, the solution can be poured down the sink, flushing the sink with warm water. Be sure to completely wash the graduated cylinder before using a different solution.
- Once you have determined the density of each solution, carefully pour a fresh 50mL portion of each solution (starting with the densest and ending with the least dense according to your calculations) into a small, clear drinking glass so the chemicals layer on top of each other. Carefully pour each of the 4 chemicals down the side of the container. Let the glass sit for 10 minutes and record the order in which they layer on the data table.
Take a photo of the glass containing the layered chemicals from Step 3. A piece of paper that includes your name, CHEM 1000 and the semester you are taking this class (for example, if you are taking this during the fall semester in 2023, write “Fall 2023” on the paper) should also appear in this photo. This information must be handwritten, legible, and large enough to be clearly read on the piece of paper. This assignment will not be graded unless this photo has been included with your submission.
Waste Disposal
- All solutions from this lab can be disposed of down the sink. Flush the sink with warm water after disposing of the chemicals.
- Wash any other glassware used with soap and water once the experiment is complete.
Assignment
Once you have completed this experiment and recorded your data on the table provided:
- Submit the completed data table for this experiment and the photo described under step 3 via the assignment folder provided on Brightspace. You will receive an automatic zero for this assignment if either of these items are missing from the assignment folder.
- Once those items have been submitted, the electronic assignment for this experiment will open for you in Module 3. Complete this assignment on Brightspace. This assignment is worth 10 points.
This page was created on July 10, 2023, and was last updated on August 3, 2023.
© Catherine Haslag 2023. All Rights Reserved.