pH of Household Chemicals
Objectives
- Utilize the pH scale to determine if something is an acid or a base.
- Explain the inherent dangers of using common household chemicals.
- Communicate laboratory experimental findings in oral and written format.
- Draw conclusions from experimental data.
Related Textbook
Before beginning this experiment, please read sections 17.6, 17.8, and 17.10 of your textbook. The textbook provides terms, concepts, and other important background information that will help you succeed on this assignment.
Some acid-base indicators can be made at home. This indicator was made from red cabbage and can be used to test the full range of the pH scale.
Introduction
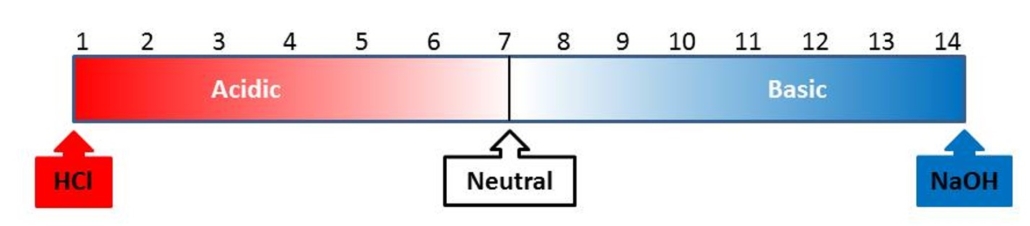
pH measures how basic or acidic something is and is measured on a scale from 1 to 14. The pH of a solution is determined by the concentration of acid, or H3O+ ions, present in solution. The higher the concentration of H3O+ ions, the lower (more acidic) the pH. Figure 1 illustrates the pH scale. Items most commonly used to determine the pH of a solution include litmus paper, pH paper, a pH indicator, or a pH meter.
Figure 1: The pH scale
The American home contains a high concentration of chemical products. There are products to clean clothes, windows, ovens, drains, floors, and people. Chemical products are used to flavor our food, thin paint, remove glue, glue things together, and make us smell good. We depend on these materials to make our lives comfortable. Many of the chemicals we used in our homes or consume as food can be classified as an acid or base.
Consumers should be aware of the dangers involved in using commercial products. Part of this knowledge comes from reading the label and knowing which ingredients might be harmful. The chemicals we use to clean our homes can cause serious injuries and even death if improperly handled. To learn more about your home’s chemicals and their dangers, watch the two videos below:
Figure 2.0: Dangers of mixing chemicals to make household cleaners.1
Figure 3.0: Can Mixing Cleaning Chemicals Kill You?2
In this experiment, we will use an indicator, pH paper, to determine the pH of common household chemicals. Indicators are generally very complex organic molecules that contain a chromophore. A chromophore is the portion of a chemical that is responsible for the chemical’s color. The structure of the chromophore changes slightly when the pH of the reaction changes, which changes the color of the pH paper. For instance, at pH = 1, the pH paper appears pink/red; at 13, the pH paper turns dark blue/purple.
Before beginning work on this experiment, please watch the video entitled How To Determine the pH of a Solution. (Figure 4.0) This video will explain how to determine the pH of a solid and liquid using pH paper.
In this week’s experiment, you will test several household chemicals to determine their pH. You will organize your data for this lab into a data table. Click below for a copy of the data table you will need for this lab. It will download to your computer. If you have trouble downloading it, check to ensure blockers are disabled.
Figure 4.0: How To Determine the pH of a Solution.
Experimental Procedure
Chemicals and Supplies
Bleach
Window Cleaner
A Fruit Juice
Milk
A bathroom cleaner
Shampoo
Hand soap
Laundry detergent
Tap water
Baking soda (sodium bicarbonate)
Vinegar (acetic acid)
Soda (Sprite or 7Up – it MUST be a clear soda)
Alka Seltzer Tablet
Three other household chemicals of your choice
DI water
Spoon
pH paper
Glass Stir Rod
Paper Towel
CAUTION: Don’t spill these materials on yourself or others. Don’t mix the household chemicals tested in this experiment as dangerous gases could be produced as a result.
- Write the chemical and commercial brand for each item tested on the data table provided.
- Tear off a piece of pH paper. Either dip the pH paper in the tested solution or place it on the watch glass and use the glass stir rod to spot the paper with the chemical.
- Record the first color the pH paper turns after spotting with the chemical being tested. Record this information on your data sheet.
- Compare the color of the pH after the addition of your chemical to the chart on the side of the container to determine the pH. Record the color and corresponding pH values on the data table provided.
- Repeat steps 2-4 with each of the chemicals being tested, being sure to clean the glass rod and watch glass between chemicals to prevent cross-contamination.
Take a photo of the pH papers after completing steps 2-4 for all the tested chemicals. To keep this photo easy, it is ok to include all the pH papers in one photo. A piece of paper that includes your name, CHEM 1000 and the semester you are taking this class (for example, if you take this during the fall semester in 2023, write “Fall 2023” on the paper) should also appear in this photo. This information must be handwritten, legible, and large enough to be clearly read on the piece of paper. This assignment will not be graded unless this photo has been included with your submission.
- Check the labels on the back of each chemical and see if there are any warnings regarding use or treatment for expose to chemical. List any warnings on your data table. If no warnings are listed, indicate so on your data table.
Waste Disposal
- Pour all chemicals down the drain one at a time, flushing the sink thoroughly with water between each chemical.
- Place any used pH paper and paper towels in the trash.
- Wash any other glassware used with soap and water once the experiment is complete.
Assignment
This assignment is designed to share and discuss your results and thoughts from completing the pH of Household Chemicals laboratory experiment. The rubric for this assignment can be viewed by clicking on the “Assessments” tab at the top of this page and selecting “Rubrics” from the drop-down menu. The rubric for this assignment is titled “pH of Household Chemicals Assignment.”
Now that you have completed the experiment and collected your data:
- Upload the photo of your experiment as described after step 5 to the assignment folder.
- Answer the following questions in a word processing program such as Word or Google sheets. Handwritten submissions for this assignment will not be accepted and will earn an automatic zero. There is no required length for this assignment; however, you need to completely answer all of the questions below using your lab results. Your paper should be written in paragraph form using complete sentences. This assignment is a short report that answers the questions below and summarizes your experimental data.
- This assignment is worth 10 points.
Do not just write the question and then provide your answer after it or number your answers. I will give you a zero if you submit your work this way. This assignment should be completed using paragraphs and complete sentences.
Below are the questions that should be answered in your short report summarizing your findings and conclusions on this experiment:
- Create a table summarizing your results using Excel, Word, or similar program. The table should be titled, numbered, logically organized, and contain all of your experimental data. Photographed data tables will not be accepted. (2 points)
- Discuss any trends observed in the pH for each group of chemicals (i.e. things we ingest, cleaners, etc.) (1 point)
- Identify any sources of error in your results. Discuss specifically how these errors would negatively impact your findings. (0.5 point)
- Check your assignment for any grammar/spelling mistakes. (0.5 point)
- Also answer the following questions in your paper:
- Any chemical with a pH below 5 or above 10 is unsafe for humans to come into direct contact with. Which of the chemicals that you tested are not safe for human contact? Are there any of these chemicals that surprise you? (2 points)
- Which of the products tested have adequate warnings concerning pH on the label? Which chemicals have inadequate warnings? Justify your opinion with data from this experiment. (2 points)
- Are there any cleaning chemicals you tested that shouldn’t be mixed together for safety reasons? You might need to look at the ingredients in the cleaning chemicals you tested to see what they are made of. (1 point)
- Will what you learned during this experiment change how you handle your household chemicals? Why or why not? Be sure to support your opinion with the data you collected from this experiment. (1 point)
References
(2) Reactions. Can Mixing Cleaning Chemicals Kill You? [Video]. Feb 19, 2019. https://youtu.be/FH1h0oWjark Accessed on July 28, 2023.
This page was created on July 28, 2023, and was last updated on December 2, 2024.
©2023 Catherine Haslag. All Rights Reserved.