Determining the Concentration of a Saturated Solution
Objectives
- Identify sources of error and explain their impact on experimental data.
- Calculate the molarity of a solution.
- Calculate the percent mass and percent volume of a solution.
- Explain how a solute dissolves in a solvent to create a solution.
- Explain intermolecular forces.
- Draw conclusions based on experimental data.
- Apply knowledge of scientific theories to problem-solving applications.
Related Textbook
Please read chapter 16 of your textbook before beginning this experiment. The textbook provides terms, concepts, and other important background information to help you complete this experiment and postlab.
Introduction
We discussed solutions and defined solute and solvent in the textbook and lecture materials. Video 1.0 also defines these terms and explains how solutions are created and the importance of solutions. Please watch the video before conducting this experiment.
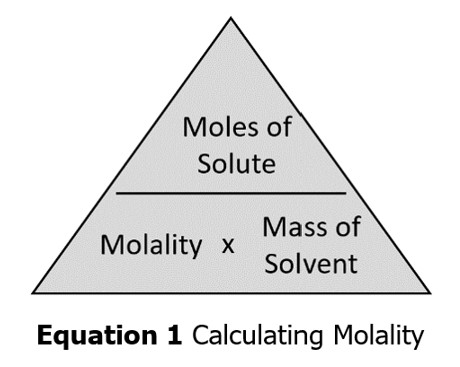
In this lab, we will apply what we have learned to create a saturated aqueous sucrose (sugar) solution in this experiment. An aqueous solution is when water is the solvent. You will then determine your solution’s molarity and percent concentration by mass. The formulas for molarity and percent concentration are provided in Equations 1 and 2. We previously worked molarity and percent concentration problems in the lecture videos posted on Brightspace.
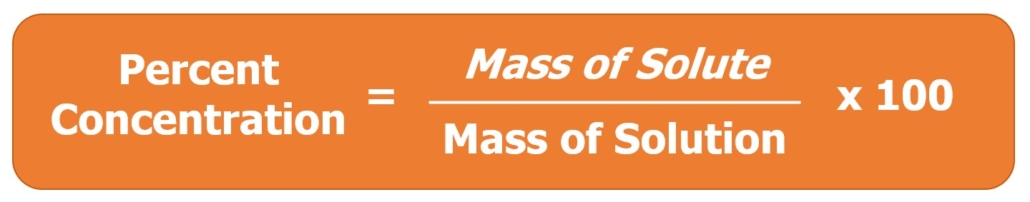
Equation 2: Calculating Percent Concentration by Mass
You will organize your data for this lab into a data table. Click below for a copy of the data table you will need for this lab. It will download to your computer. If you have trouble downloading it, check to ensure blockers are disabled.
Determining the Concentration of a Saturated Solution Data Table
Video 1: What is a solution and how are they created.
Experimental Procedure
Experimental Materials
Sucrose (C12H22O11)
DI water
Clear glass
Digital Balance
10 & 100mL graduated cylinders
Thermometer
weigh boats
Dark piece of paper or cloth (black if possible)
- Measure between 50 and 60mL of water using a graduated cylinder. Record the exact volume (to 0.1mL) and temperature of the water on the data table under Trial 1.
- Pour the water into the clear glass. Place the clear glass on the dark paper/fabric. Since the solute (sucrose) is white, you can see it more easily against the contrast of the dark paper/fabric when it no longer dissolves in water.
- Add your solute, approximately 4g at a time, to the glass of DI water. Record exactly how much solute you add to the DI water (to 0.1g) on the data table. Gently swirl the solution to dissolve the solute, careful not to allow any of the solution to spill from the glass. If the solute completely dissolves, add another approximately 4g of solute to the DI water, recording the amount added (to 0.1g) on the data table. Swirl again to dissolve the sucrose. Continue this addition of solute/swirling until you see undissolved solute (white crystals) collecting on the bottom of the glass. When you see this solute forming, this means your solution is saturated.
- Now that your solution is saturated, add 1.0mL of DI water at a time, swirling well, until all the solute on the bottom is completely dissolved. Record the added volume of water on the data table under Trial 1.
- Use the density formula to determine the mass of all of the water used to create this solution. Record this mass on the data table under trial 1.
- Repeat Steps 1 to 5 and record information under Trial 2 on the data table.
Waste Disposal
- All solutions from this lab can be disposed of down the sink. Flush the sink with warm water after disposing of the chemicals.
- Wash any other glassware used with soap and water once the experiment is complete.
Assignment
Now that you have completed this experiment and recorded your data:
- Submit the completed data table for this experiment. You do not need to submit an experiment photo for this lab.
- Complete all the questions below. Please number your answers to each question so I can clearly and easily follow your work. Your answers MUST be handwritten. Typed work for the postlab questions will not be accepted. This assignment is worth 10 points.
Postlab Questions
- Calculate the molarity of the saturated solutions you created in Trials 1 and 2. You must show all of your calculations to receive credit for this problem. (3 points)
- Calculate the percent concentration by mass of the saturated solutions you created in Trials 1 and 2. You must show all of your calculations to receive credit for this problem. (2 points)
- You accidentally bump the glass and spill some water before adding sucrose. How will this impact your experimentally determined concentration? (1 point)
- What type of intermolecular forces occur between the water molecules in the solution you created? (1 point)
- What type of intermolecular forces occur between the water and sucrose molecules in the solution you created? (1 point)
- Based on the solubility of sucrose in water, is sucrose a polar or nonpolar molecule? Explain your reasoning. (2 points)
References
Binel, T.H., Mueldener, C., and Smiley, T. C. Journal of Chemical Education [Online] 1994, 71 (9), 792. DOI: 10.1021/ed071p792 (accessed February 1, 2024).
Lechtanski, V. L. Determining the Molarity of a Saturated Solution. In Inquiry-Based Experiments in Chemistry. Oxford University Press, 2000, pp 111-117.
The Science Basement. How Solubility and Dissolving Work [Video]. YouTube, Nov 25, 2021. https://youtu.be/JGkmGQ89_SE?si=_I71qoBbtvTZGVXj (accessed Feb 8, 2024).
This page was created on February 1, 2024, and was last updated on February 8, 2024.
©2024 Catherine Haslag. All Rights Reserved.