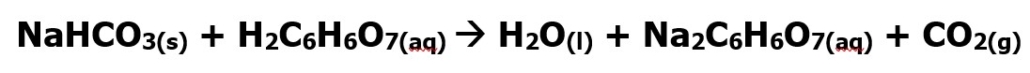Stoichiometric Determination of Carbon Dioxide Produced from Alka Seltzer®
Objectives
- Calculate the theoretical yield and percent yield for a product in a chemical reaction.
- Apply knowledge of scientific theories to problem-solving applications.
- Identify sources of error and explain their impact on experimental data.
- Determine if a process is exothermic or endothermic.
- Draw conclusions based on experimental data.
- Utilize the pH scale to determine if something is an acid or a base.
Related Textbook
Please read chapter 11 of your textbook before beginning this activity. The textbook provides terms, concepts, and other important background information to help you complete this assignment.
Introduction
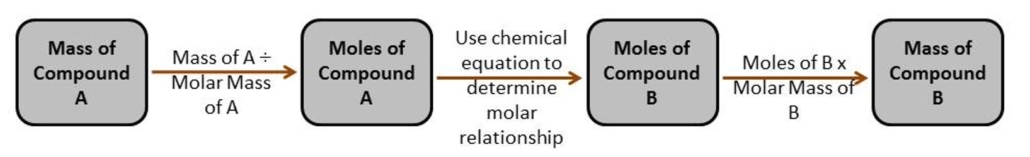
If you know the overall chemical equation for a reaction, the amount of product that can be theoretically produced from a known amount of reactant can be determined. This process is referred to as stoichiometry. Figure 1.o provided below illustrates a flow chart for solving stoichiometry problems:
Figure 1: Flow chart for completing stoichiometric calculations.
The maximum amount of product that can be produced is determined by using the reactant that will be consumed completely first. This chemical is called the limiting reagent. The limiting reagent can be determined mathematically or using observations made during the reaction.
In this experiment, you will react Alka Seltzer® which contains sodium bicarbonate (baking soda) with citric acid producing carbon dioxide. This will give you the experimental amount of carbon dioxide produced in this reaction. You will then calculate the theoretical amount of carbon dioxide that would be produced based on the amount of sodium bicarbonate and citric acid present in a dose of Alka Seltzer®. This information can be obtained from the side of the box under Active Ingredients. Please note that the amount shown on the box may be per tablet, not per dose (2 tablets). Since you don’t know if the citric acid or sodium bicarbonate is the limiting reagent, you will need to determine that prior to determining the theoretical yield. This process is just like the calculations we completed in the stoichiometry and limiting reagents lectures.
The overall chemical equation for this reaction is provided below. The small letters that appear after the chemical formula indicate the phase of matter. S stands for solid, aq for aqueous (dissolved in water), l stands for liquid, and g means gas. Be sure to balance the equation below before beginning any calculations.
Figure 2: Reaction of sodium bicarbonate and citric acid.
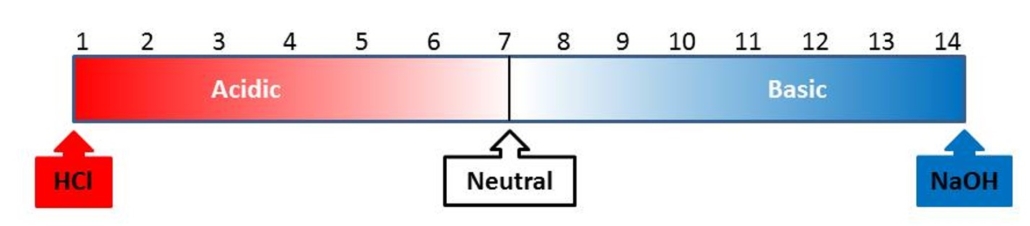
One of the observations you will be making during this experiment will be about the pH of the solution. pH is the measure of how basic or acidic something is and is measured on a scale from 1 to 14. Figure 3 below illustrates the pH scale.
Figure 3: The pH scale
The reaction between sodium bicarbonate and citric acid is an acid-base reaction. When the solution is basic (pH between 7 and 14), this means all of the citric acid has been reacted, and excess sodium bicarbonate remains. When the solution is acid (pH between 1 and 7), all sodium bicarbonate has been reacted, and excess citric acid remains.
We will use an indicator, pH paper, to determine if a pH change has occurred. Indicators are generally very complex organic molecules that contain a chromophore. A chromophore is the portion of a chemical that is responsible for the chemical’s color. The structure of the chromophore changes slightly when the pH of the reaction changes, which changes the color of the solution. Instructions on how to use pH paper to determine the pH of a solution are provided in Figure 4.
The reaction between sodium bicarbonate and citric acid is an acid-base reaction. When the solution is basic (pH between 7 and 14), this means all of the citric acid has been reacted, and excess sodium bicarbonate remains. When the solution is acid (pH between 1 and 7), all sodium bicarbonate has been reacted, and excess citric acid remains.
We will use an indicator, pH paper, to determine if a pH change has occurred. Indicators are generally very complex organic molecules that contain a chromophore. A chromophore is the portion of a chemical that is responsible for the chemical’s color. The structure of the chromophore changes slightly when the pH of the reaction changes, which changes the color of the solution. Instructions on how to use pH paper to determine the pH of a solution are provided in Figure 4.
Figure 4: How to use pH paper to determine the pH of a solution.
Percent Yield
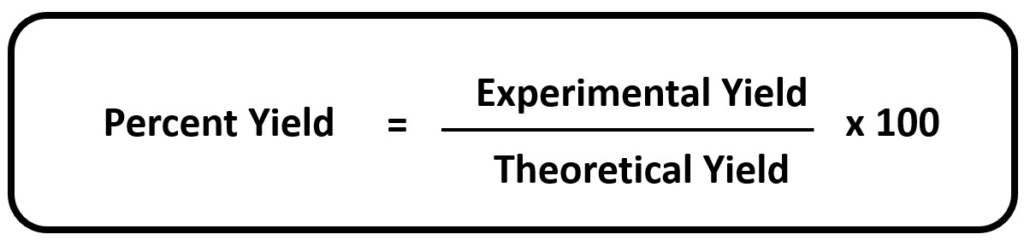
The percent yield for each reaction will also be calculated to determine how close the experimentally produced product is to the theoretical yield. The percent yield is calculated by dividing the actual yield (the amount experimentally produced) by the theoretical yield and multiplying by 100%. The formula for determining percent yield is outlined in Figure 5. The higher the percent yield, the closer the experimental yield is to the theoretical yield.
Figure 5: Percent Yield Formula
Do not be concerned if the percent yield is not equal to the theoretical yield. Human error will diminish the amount of product that these reactions will produce. In addition, not all reactions will produce the calculated theoretical yield. For some reactions, a 20% or 30% yield is all that can ever be obtained. However, the percent yield should never exceed 100% as matter would have been created, and that can never happen (remember the Law of Conservation of Matter).
You will organize your data for this lab into a data table. Click below for a copy of the data table you will need for this lab. It will download to your computer. If you have trouble downloading it, check to ensure blockers are disabled.
Safety Concerns
There are no safety hazards in this experiment.
Do not ingest the Alka Seltzer®. It is considered a laboratory chemical and should not be consumed as a part of this lab.
Experimental Procedure
Chemicals and Supplies
Digital Balance
Digital Thermometer
2 Packets of Alka Seltzer® (regular, NOT the cold/flu version)
DI Water
pH Strips
Weigh boats
100mL Graduated Cylinder
500mL Beaker
Glass stir rod
Figure 6: Where to find the active ingredients on the side of the Alka Seltzer® label. If your label lists these as inactive ingredients, then use the values shown in this photo as your initial amounts of sodium bicarbonate and citric acid reacted.
- Open a packet of Alka Seltzer®. Obtain the mass of 2 tablets and record this information on the data table under Trial 1.
NOTE: To properly measure the mass of the Alka Seltzer® tablets, place a weigh boat on the digital balance and “TARE” the balance according to the directions provided with your balance. This tells the balance to ignore the mass of the weigh boat and only measure the mass of the chemical. Next, add the Alka Seltzer® tablets to the weigh boat and record the mass on the data table.
- Write down the amount of active ingredients (the sodium bicarbonate and citric acid, found on the side of the box, see Figure 6.0) for both tablets on the data table. This will be the same for both trials.
- Measure 200mL of DI water and add it to the 500mL beaker. Obtain the mass of the beaker and water. Record this information on the data table under Trial 1.
- Obtain the initial temperature and pH of the DI water. Record this information on the data table under Trial 1.
- Add the Alka Seltzer® tablets to the DI water 1 at a time, waiting until the reaction is completed for the first tablet before adding the second tablet. This will prevent the reaction from overflowing the beaker.
NOTE: If the reaction overflows the beaker, you must start this trial over. The reaction must be contained inside the beaker, allowing only any gas produced to escape.
- Once the reaction has stopped, use the glass stir rod to stir the contents of the beaker, being sure to knock all of the carbon dioxide bubbles from the sides and bottom of the beaker.
- Measure the pH and temperature of the contents of the beaker. Record this on the data table under Trial 1.
- Obtain the final mass of the beaker and its contents. Record this information on the data tablee under Trial 1.
Take a photo of the beaker on the balance with the beaker on it from step 6. A piece of paper that includes the trial number, your name, CHEM 1000, and the semester you are taking this class (for example, if you are taking this during the fall semester in 2023, write “Fall 2023” on the paper) should also appear in this photo. This information must be handwritten, legible, and large enough to be clearly read on the piece of paper. The assignment tied to this laboratory will not be graded unless this photo has been submitted to the assignment folder on Brightspace along with the rest of the assignment for this experiment.
- Repeat steps 1 through 8 of this experiment, recording the information on the Data Table under Trial 2. Be sure also to take a photo for this trial, as outlined at the end of step 8.
Waste Disposal
- Pour all chemicals down the drain, flushing the sink thoroughly with water.
- Place all used pH paper and paper towels in the trash.
- Wash any other glassware used with soap and water once the experiment is complete.
Assignment
Now that you have completed the experiment and recorded your data in the data table:
- Use the data from your experiment to answer the post-lab questions included below. All calculations and questions MUST be handwritten. Typed submissions for this assignment will not be accepted. Please be sure to number your answers so they are easy to follow.
- Submit the data table, the post-lab questions, and the experimental photos to the assignment folder on Brightspace. You will receive an automatic zero for this assignment if the experimental photo or data are missing from your submission.
- This assignment is worth 10 points.
Postlab Questions
- Calculate the theoretical yield for the amount of carbon dioxide produced from the amount of sodium bicarbonate and citric acid reported on the side of the Alka Seltzer® Show ALL calculations, include units, and circle your final answer. You will earn a zero for this question if all of your work isn’t shown. The equation for this reaction is provided in the introduction of this experiment. (5 points)
- Determine the average amount of carbon dioxide produced during your two trials. Using this value as the experimental value, determine the percent yield for this reaction. If the value of your experimental yield is larger than your calculated theoretical yield(from Question 1), explain why this could have happened. (1 point)
- What evidence of chemical reaction did you observe during this experiment? List all evidence of a chemical reaction that occurred. (1 point)
- Is this reaction endothermic or exothermic? What evidence from your experiment do you have to support your conclusion? (1 point)
- What is one potential source of error from this experiment (other than human error)? Explain specifically how this error would affect your results. (2 points)
This page was created on July 12, 2023, and was last updated on October 23, 2024.
©2023 Catherine Haslag. All Rights Reserved.